Abstract
The lipopeptide antimicrobial daptomycin was administered intravenously at a dose of 4 mg/kg of body weight to seven healthy male volunteers. The concentrations of daptomycin in plasma, cantharidin-induced inflammatory fluid, and urine were measured by a microbiological assay. The mean ± standard deviation peak concentrations in plasma and inflammatory fluid were 77.5 ± 8.3 and 27.6 ± 9.5 μg/ml, respectively; the mean terminal elimination half-lives were 7.74 and 13.2 h, respectively. The overall penetration of total drug into the inflammatory fluid (measured by ratio of the area under the concentration-time curve from 0 to 24 h for inflammatory fluid compared with that for plasma) was 68.4%. The mean urinary recovery over 24 h was 59.7%.
Daptomycin is a cyclic lipopeptide antimicrobial with in vitro activity against gram-positive organisms including vancomycin-resistant enterococci, methicillin-resistant staphylococci, and “heterodrug-resistant” glycopeptide-resistant Staphylococcus aureus (M. D. Appleman and D. M. Citron, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2291, p. 183, 2000; E. J. C. Goldstein and D. M. Citron, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2293, p. 183, 2000). In vitro studies to date have shown that daptomycin is rapidly bactericidal, with treatment with daptomycin resulting in greater than 3 log reductions in 8 h for methicillin-resistant S. aureus, vancomycin-resistant enterococci, and glycopeptide-resistant S. aureus (1). Daptomycin is being investigated against complicated skin and skin structure infections in two large phase III trials by use of a dose of 4 mg/kg of body weight every 24 h. There is little recent published information on the pharmacokinetics of this compound; hence, the aim of the present study was to investigate the pharmacokinetics of daptomycin following the administration of a single intravenous dose of 4 mg/kg. In addition, a cantharidin-induced inflammatory blister technique was used to assess the penetration of this compound into an inflammatory exudate (2).
MATERIALS AND METHODS
Seven healthy male volunteers (age range, 21 to 28 years; mean weight, 78.5 kg; weight range, 70.1 to 95.2 kg; mean height, 179.8 cm; height range, 173 to 195 cm) gave written informed consent following hospital ethical committee approval of the protocol. Exclusion criteria included atopy, liver and renal disease, and significant central nervous system pathology.
All volunteers underwent a full medical history examination and routine hematological and biochemical investigations. On the evening prior to the study two 0.2% cantharidin-impregnated plasters (1 by 1 cm) were placed on each subject’s forearm to induce inflammatory blister formation. The volunteers received only clear fluids in the 8 h prior to administration of the plasters.
The volunteers received daptomycin at a dose of 4 mg/kg of body weight administered in 50 ml of normal saline over 30 min. For the next 2 h only clear fluids were taken by mouth, after which a light diet was allowed. Blood samples were obtained at time zero (at the beginning of infusion), 0.25 h (halfway through the infusion), 0.5 h (at the end of the infusion), and 0.58, 0.75, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after the infusion. Blister fluid was sampled with a fine needle at 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h. The blister was then resealed by spraying it with a plastic dressing aerosol. Urine was collected over 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after the initiation of the infusion.
Drug analysis.
The blood samples were centrifuged, and the plasma was collected and assayed, in triplicate, within 1 h. Plasma, inflammatory fluid, and urine daptomycin concentrations were measured by a microbiological assay with Isotonic Sensitivity Test agar (Mast, Merseyside, United Kingdom) supplemented with 50 mg of Ca2+ per liter. The indicator organism was S. aureus F1445 (from the organism collection of the Department of Microbiology, City Hospital NHS Trust, Birmingham, United Kingdom), which was incorporated into the agar in order to achieve semiconfluent growth. The daptomycin standard concentration range was 2 to 32 mg/liter, and the lower limit of sensitivity of the assay was 1 mg/liter. Calibrators were prepared in human serum, 70% serum (in buffer at pH 7), and phosphate buffer (pH 7) for the plasma, inflammatory fluid, and urine samples, respectively. Incubation was for 24 h, after which the assay plates were read with an image analyzer (Image Associates, Teme, United Kingdom). The interassay coefficients of variation (for the internal controls) were 6.3, 7.8, and 7.2% for plasma, inflammatory fluid, and urine, respectively; and the intra-assay regression analysis of quality assurance samples yielded r2 values of 0.959, 0.82, and 0.99 for the three types of samples, respectively. All samples were processed within 1 h of collection.
Pharmacokinetic analysis.
Plasma daptomycin concentration data were analyzed by compartmental and model-independent methods with WinNonLin software (version 3.0; Pharsight Corp., Mountain View, Calif.), and the inflammatory fluid daptomycin concentration data were analyzed by model-independent means. Determination of the area under the concentration-time curve (AUC) for inflammatory fluid used a log-linear method; for the AUC for plasma the linear trapezoidal rule was applied. The following were calculated: the maximum concentration (Cmax) in plasma or inflammatory fluid and the time to Cmax (Tmax), the AUCs for plasma and inflammatory fluid over the dosing interval of 0 to 24 h (AUC0–24), the AUC extrapolated to infinity (AUC0–∞), the terminal linear elimination half-life (t1/2), the clearance from plasma (CLplasma), the volume of distribution of daptomycin in the central compartment (V1), the volume of distribution based on the terminal phase (Vz), the volume of distribution at steady state (Vss), the mean residence time in plasma (MRT), the percent excreted unchanged in urine over 24 h (Ae), and the percent penetration into inflammatory fluid (which is the ratio of the AUC0–24 for inflammatory fluid compared with that for plasma).
RESULTS
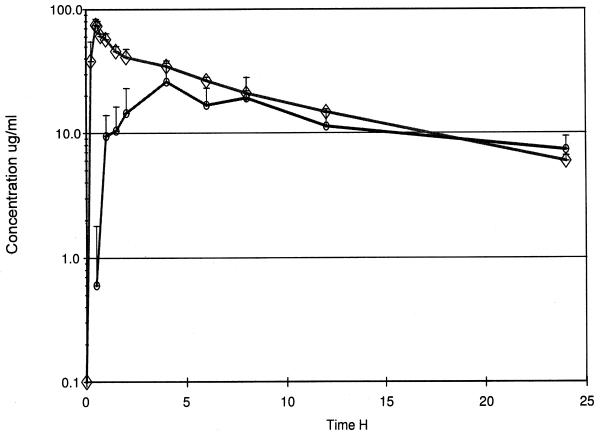
The mean concentrations of daptomycin in plasma and the inflammatory fluid following administration of the drug at 4 mg/kg are shown in Fig. 1, and the derived pharmacokinetic parameters are shown in Tables 1 and 2. These data were obtained for six of the seven volunteers, with one volunteer having to be excluded due to an adverse event (see below). There was good agreement between the data generated by compartmental and noncompartmental analyses. V1, normalized to body weight, corresponds to the volume of distribution for plasma (mean ± standard deviation [SD], 0.043 ± 0.011 liter/kg); Vss did not differ significantly from Vz. When these values were normalized to body weight, they reflected the Vss and Vz values for plasma and interstitial fluid (0.082 ± 0.013 liter/kg).
FIG. 1.
Mean concentrations in plasma (◊) and inflammatory fluid (○) following intravenous administration of a 4-mg/kg dose of daptomycin over 30 min (vertical bars indicate SDs).
TABLE 1.
Daptomycin pharmacokinetic parameters following intravenous infusion of a 4-mg/kg dose over 30 min
| Subject | Wt (kg) | AUC0–24 (μg · h/ml) | AUC0–∞ (μg · h/ml) | Cmax (μg/ml) | t1/2 (h) | CLplasma (ml/min) | V1a (ml) | Vz (ml) | Vss (ml) | MRT (h) | Ae (% of dose) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78.2 | 464.3 | 513.2 | 81.5 | 7.13 | 10.13 | 2,929 | 5,973 | 5,797 | 9.89 | 47.0 |
| 3 | 80.7 | 452.9 | 533.4 | 63 | 8.76 | 9.95 | 4,518 | 7,135 | 7,107 | 12.50 | 64.2 |
| 4 | 73.3 | 460.3 | 528.5 | 73 | 8.15 | 9.84 | 2,336 | 6,708 | 5,978 | 11.42 | —b |
| 5 | 70.1 | 470.1 | 529.8 | 84.5 | 7.69 | 9.82 | 2,189 | 6,267 | 5,029 | 10.70 | 50.5 |
| 6 | 73.5 | 462.7 | 521.5 | 79 | 7.57 | 9.92 | 3,941 | 6,393 | 6,318 | 10.89 | 69.4 |
| 7 | 95.2 | 497.6 | 551.7 | 84 | 7.13 | 9.35 | 4,607 | 5,624 | 6,111 | 10.22 | 67.3 |
| Mean | 78.5 | 468.0 | 529.7 | 77.5 | 7.74 | 9.84 | 3,420 | 6,350.0 | 6,057 | 10.94 | 59.7 |
| SD | 9.0 | 15.6 | 13.0 | 8.3 | 0.63 | 0.26 | 1,070 | 533.4 | 679 | 0.93 | 10.2 |
| CVc (%) | 11.53 | 3.32 | 2.45 | 10.64 | 8.14 | 2.66 | 31.5 | 8.4 | 11.2 | 8.5 | 17.1 |
Value obtained by compartmental analysis.
—, no sample was obtained.
TABLE 2.
Daptomycin pharmacokinetics in inflammatory fluid following administration of a 4-mg/kg dose
| Subject | AUC0–24 (μg · h/ml) | Cmax (μg/ml) | Tmax(h) | t1/2(h) | % Penetration |
|---|---|---|---|---|---|
| 1 | 345 | 40.6 | 4 | 13.2 | 74.3 |
| 2 | 447 | 34.0 | 4 | 8.49 | 98.7 |
| 4 | 373.3 | 30.0 | 4 | 6.32 | 81.1 |
| 5 | 263.3 | 24.5 | 2 | 12.7 | 56.0 |
| 6 | 256.9 | 23.2 | 4 | 30.0 | 55.5 |
| 7 | 223.9 | 13.2 | 4 | 30.9 | 45.0 |
| Mean | 318.2 | 27.6 | 3.67 | 17.3 | 68.4 |
| SD | 84.9 | 9.5 | 0.81 | 11.3 | 19.9 |
| CVa (%) | 26.7 | 34.4 | 22.2 | 65.3 | 29.1 |
CV, coefficient of variation.
The mean plasma daptomycin Cmax was 77.5 μg/ml at the end of the infusion period. The mean t1/2 from plasma was 7.74 h, with relatively little individual variation (SD, 0.63 h). The mean AUC0–24 was 468.0 μg · h/ml. The mean AUC0–24 was equal to 88% of the AUC0–∞.
Daptomycin penetrated the inflammatory exudate moderately rapidly, with the mean concentrations at 1 and 2 h being 9.4 and 14.5 μg/ml, respectively. The mean Tmax was 3.7 h when the mean Cmax in inflammatory fluid was 27.6 μg/ml. The t1/2 of daptomycin from the inflammatory exudate was highly variable, ranging from 6.3 to 32 h, with the mean being 17.3 h. The mean AUC0–24 for this fluid was 318.2 μg · h/ml. When the penetration is compared by determination of the ratio of the AUC0–24 for inflammatory fluid compared with that for plasma, a mean value of 68.4% (coefficient of variation, 29.1%) was obtained.
The mean ± SD urinary elimination of the drug from five of the six volunteers was 59.7% ± 10.2%; the set of data for one volunteer was not included as no urine was said to have been collected from 0 to 12 h postadministration. The biochemical and hematological parameters studied showed no drug-related abnormalities.
Two volunteers experienced adverse events. One volunteer was withdrawn from the study because of serious diarrhea followed by vomiting starting approximately 1.5 h after the end of the intravenous infusion. He required intravenous metoclopramide and fluids; the symptoms subsided within the following 5 to 6 h. The second volunteer experienced a milder incident of a similar nature but continued in the study.
The severity of the gastrointestinal symptoms observed in the two volunteers mentioned above was unexpected and has not been seen in other studies in patients or healthy volunteers with daptomycin at doses up to 8 mg/kg/day for 14 days. Gastrointestinal adverse effects, while observed, have not been common, occurring at rates similar to those for comparative antibacterial agents (Barry Dvorchik, personal communication).
DISCUSSION
There are limited published data on the pharmacokinetics of daptomycin. A linear dose-response has been demonstrated for 1 to 6 mg/kg given intravenously (3), with a mean t1/2 from plasma of 8.56 h, close to the value of 7.74 h found in the present study. The earlier study demonstrated a AUC0–∞ for plasma of 382 μg · h/ml, whereas our value was higher (537 μg · h/ml). This difference can probably be accounted for by our observation of a higher Cmax (77.5 μg/ml), whereas the value in the earlier study was 52.3 μg/ml. This may well be related to differences in the preparation of the daptomycin solution.
Daptomycin penetrated into the inflammatory fluid fairly rapidly; the Tmax for inflammatory fluid was about 3 h later than that for plasma. The mean penetration into this inflammatory exudate was 68.4%. The level of binding of daptomycin to serum proteins is 90% (mean). The protein content of inflammatory exudate is about 70% of that of plasma. Thus, the penetration of daptomycin into this inflammatory exudate most likely reflects these two factors, and differences in the protein content of the inflammatory exudate compared with that of plasma may explain differences in the observed penetration. The high level of protein binding of daptomycin and the lack of protein in the interstitial fluid were probably the most likely explanations for the fact that the apparent volumes of distribution of daptomycin (Vz or Vss) were lower than the estimates of the extracellular fluid volume (0.157 to 0.187 liter/kg).
REFERENCES
- 1.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise, R., A. P. Gillett, B. Cadge, R. Durham, and S. Baker. 1980. The influence of protein binding upon tissue fluid levels of six β lactam antibiotics. J. Infect. Dis. 142:77–82. [DOI] [PubMed] [Google Scholar]
- 3.Woodworth, J. R., E. H. Nyhart, G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]