Abstract
We report on a case of a postneurosurgical meningitis due to ceftriaxone-susceptible Proteus penneri, with selection of a ceftriaxone-resistant isolate following treatment with ceftriaxone. The isolates presented identical patterns by pulsed-field gel electrophoresis and produced a single β-lactamase named HugA with an isoelectric point of 6.7. The ceftriaxone-resistant isolate hyperproduced the β-lactamase (increase in the level of production, about 90-fold). The sequences of the hugA β-lactamase gene and its regulator, hugR, were identical in both P. penneri strains and had 85.96% homology with those of Proteus vulgaris. The HugA β-lactamase belongs to molecular class A, and the transcriptional regulator HugR belongs to the LysR family.
The clinical significance of Proteus penneri, described in 1982 as a new species (9) and previously known as Proteus vulgaris indole negative, is poorly documented (13, 17). Like P. vulgaris, P. penneri is naturally resistant to ampicillin, narrow-spectrum cephalosporins, and cefuroxime by the production of a β-lactamase commonly named cefuroximase (7, 15, 16). Only the β-lactamase of P. vulgaris has been sequenced and characterized (6, 11). Named CumA (6), it belongs to molecular class A and functional group 2 e (4) of the β-lactamases and is regulated by a system common to class C of the β-lactamases (6, 11).
We report on a postneurosurgical infection due to P. penneri complicated by the emergence of a ceftriaxone-resistant isolate during treatment with ceftriaxone. Our purpose was to characterize the isolates of P. penneri and to understand the emergence of a ceftriaxone-resistant isolate of P. penneri by sequencing the β-lactamase gene and its regulator.
Case report.
After a severe cranial trauma, a reconstruction of the frontal and cranial basis with a muscular homograft and prosthetic material was performed in a 34-year-old patient. Four days later, because of suspected bronchopneumonia while the patient was under ventilation, a bronchoalveolar lavage (BAL) showed the presence of Staphylococcus aureus at 105 CFU/ml and P. penneri (isolate S08) at 104 CFU/ml. The bronchopneumonia resolved spontaneously. At day 22 a cerebral computed tomography scan followed by neurosurgical intervention showed a basal frontal breach and a cerebral abscess containing a pure culture of P. penneri (isolate S29) susceptible to ceftriaxone. Treatment with ceftriaxone (2 g twice a day [b.i.d.]), amikacin (500 mg b.i.d.), and metronidazole was instituted. Twelve days later, a P. penneri strain resistant to ceftriaxone (isolate R15) was isolated from the cerebrospinal fluid (CSF). A third neurosurgical intervention showed a frontal epidural infection and osteomyelitis due to a P. penneri strain resistant to ceftriaxone. With treatment with imipenem (500 mg four times a day) and gentamicin (120 mg b.i.d.) for 6 weeks, the patient healed without neurologic sequelae.
The isolates (S08 from BAL fluid, S29 and R15 from CSF) were identified as P. penneri with the API Rapid ID 32E gallery (bioMérieux, Marcy l’Etoile, France). They showed completely identical banding patterns by molecular typing, performed by pulsed-field gel electrophoresis after digestion with SfiI and NotI (New England Biolabs, Beverly, Mass.).
The results of antimicrobial susceptibility testing, performed according to the guidelines of NCCLS (18), are reported in Table 1. The isolate from CSF, P. penneri R15, was resistant to ceftriaxone; and synergy between the amoxicillin-clavulanic acid disk and the ceftriaxone disk and/or the cefepime disk was presented for this isolate, whereas such synergy was not found for isolate P. penneri S29, susceptible to ceftriaxone. According Le Comité de l’Antibiogramme of the French Society for Microbiology (1), this pattern of synergy for P. penneri suggests hyperproduction of a β-lactamase instead of production of an extended-spectrum β-lactamase.
TABLE 1.
MICs for two isolates of P. penneri, S29 and R15
| Antimicrobial agent | MIC (μg/ml)
|
|
|---|---|---|
| Isolate S29 | Isolate R15 | |
| Ampicillin | >256 | >256 |
| Cephalothin | >256 | >256 |
| Cefuroxime | >256 | >256 |
| Cefoxitin | 8 | 8 |
| Piperacillin | 2 | 128 |
| Piperacillin + tazobactam | 0.5 | 2 |
| Ceftriaxone | 0.125 | 64 |
| Cefotaxime | 0.25 | 16 |
| Ceftazidime | 0.125 | 2 |
| Cefpirome | 0.25 | 32 |
| Cefepime | 0.125 | 2 |
| Imipenem | 0.25 | 1 |
| Meropenem | 0.06 | 0.125 |
Specific activities of β-lactamase.
Bacterial strains were obtained from 4-liter cultures grown in brain heart infusion broth. Cells were harvested by centrifugation at 5,800 × g for 30 min. The pellets (about 12 g [wet weight]) were washed by resuspension in 24 ml of a 0.1 M NaCl solution and centrifuged as described above, and the supernatant was discarded. Then the pellets were resuspended in 24 ml of the same solution and lysed by ultrasonic treatment. The crude extracts were cleared by centrifugation at 48,000 × g for 30 min at 4°C. These extracts were used for specific activity determinations by computerized microacidimetry (14). P. penneri S29 and R15 both produced a single β-lactamase of pI 6.7, as determined by analytical isoelectric focusing, but the β-lactamases had different specific activities (2). These activities were determined with cephalothin as the substrate for crude extracts. The resistant strain increased its level of β-lactamase production 91-fold compared with that for the susceptible strain, with production of 8.5 ± 1 and 0.093 ± 0.01 U/mg, respectively. The kinetic parameters of the highly purified (>97%) β-lactamase from P. penneri R15 (Table 2) showed high degrees of activity for most cephalosporins, with kcat values ranging from 376 s−1 for cefamandole to 65 s−1 for ceftriaxone. The level of hydrolysis of ceftazidime was very low (0.5 s−1), a value comparable to that for ticarcillin. The enzyme was inhibited by clavulanic acid and tazobactam, with Ki values of 0.15 and 0.12 μM, respectively. In terms of kcat/Km values, cephalothin appears to be the best substrate and ceftriaxone is hydrolyzed about two times more rapidly than cefotaxime.
TABLE 2.
Kinetic parameters for the β-lactamase purified from P. penneri R15 and P. vulgaris Ro104
| Antimicrobial agent |
P. penneri
|
P. vulgarisa
|
|||||
|---|---|---|---|---|---|---|---|
| kcatb (s−1) | Kmb (μM) | kcat/Km (s−1 mM−1) | kcatb (s−1) | Kmb (μM) | kcat/Km (s−1 mM−1) | ||
| Benzylpenicillin | 10 | 6.0 | 1,700 | 19 | 4.0 | 4,875 | |
| Amoxicillin | 10 | 14.7 | 700 | 20 | 12.3 | 1,626 | |
| Ticarcillinc | 0.5 | 5.0 | 100 | 1.1 | 4.6 | 239 | |
| Cephalothin | 260 | 28 | 9,300 | 695 | 26 | 26,700 | |
| Cephaloridine | 210 | 83 | 2,500 | 496 | 92 | 6,500 | |
| Cefuroxime | 165 | 190 | 860 | 195 | 40 | 4,900 | |
| Cefamandole | 376 | 320 | 1,175 | 4,480 | 190 | 23,600 | |
| Cefotaxime | 60 | 950 | 63 | 75 | 170 | 447 | |
| Ceftriaxone | 65 | 500 | 130 | 79 | 45 | 1,800 | |
| Ceftazidimec | 0.5 | 350 | 1.4 | 1.0 | 170 | 6.0 | |
| Cefoxitinc | 5 | 2.3 | |||||
| Moxalactamc | 10 | 5.1 | |||||
| Imipenemc | 6 | NDd | ND | ND | |||
Data were taken from Peduzzi et al. (19).
For kcat values standard deviations (SD) are lower than 10%, and for Km values, standard deviations are lower than 20%.
For compounds with poor (ticarcillin, ceftazidime) or undetectable (cefoxitin, moxalactam, imipenem) hydrolytic activities, Ki instead of Km values were determined by competition procedures with cephalothin as the reporter substrate.
ND, not determined.
Sequencing of the β-lactamase.
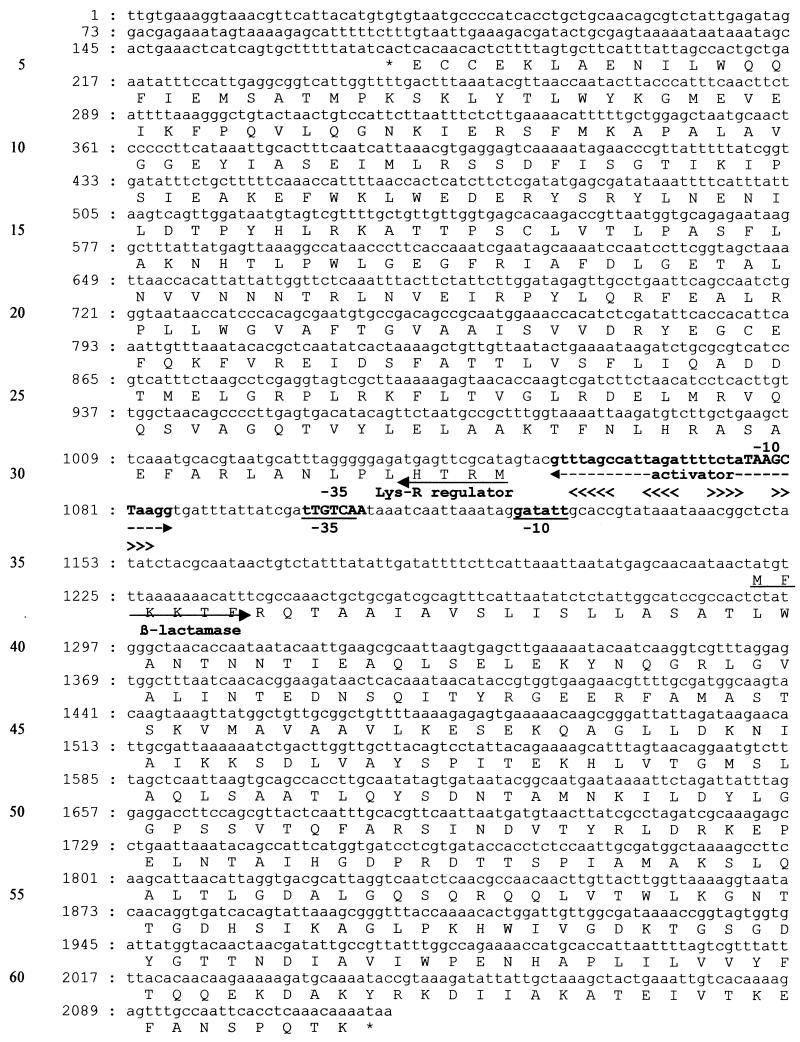
The DNAs of P. penneri S29 and R15 were extracted by mechanical lysis and amplified with five pairs of primers chosen according to the sequences of the β-lactamase gene of P. vulgaris (cumA gene) and its transcriptional regulator gene (cumR gene) (6) (pair 1, 5′-GTCAACTCGTGAAGGGAA-3′ and 5′-TTTTTGACTCCTCACGTT-3′; pair 2, 5′-AGCGCTTCATTCATTAGCCAT-3′ and 5′-TGAAGCTTCAGCAAGGCACCT-3′; pair 3, 5′-CGATCTTCTAACATCCTC-3′ and 5′-AAGCTCACTTAATTGCGC-3′; pair 4, 5′-TATGAGCAACAATGACTATG-3′ and 5′-ACCACTGCCAGTTTTATC-3′; pair 5, 5′-TCAAGCATTAACATTAGG-3′ and 5′-TATTTTGTTTGAGGTGAA-3′). Three independent PCR products from each amplification were then analyzed by direct cycle sequencing with ABI dye terminator TaqFSkit (Applied Biosystems, Rotkreuz, Switzerland) and the ABI 373A apparatus (Applied Biosystems). Both strands of DNA were sequenced. The sequences coding for the β-lactamase, named HugA, and an LysR-type regulator, named HugR, were identical in the susceptible and the resistant isolates (Fig. 1). The gene sequences had 85.96% homology with those of P. vulgaris. The deduced amino acid sequence of the β-lactamases of the two isolates of P. penneri showed structural features typical of a class A β-lactamase (12): active-site serine S*-T-S-K and its limiting elements S-D-N, E-P-E-L-N, and K-T-G. A serine was present at position 237 (21). The protein alignments of β-lactamase HugA of P. penneri and the CumA β-lactamase of P. vulgaris (6) showed great similarities, with 282 of 298 (94.6%) residues being identical.
FIG. 1.
DNA sequence and translation of the β-lactamase gene hugA and its regulator, hugR. The start codons and the beginnings of proteins are indicated by arrows, and the stop codons are indicated by asterisks. The −35 and −10 promoter sequences of β-lactamase are underlined, whereas those of the regulator are in capital letters. A 31-base activator is indicated; it contains two inverted sequences, identified by the two pairs of arrowheads (<<<< and >>>>). Sequences are similar to those noticed by Ishiguro and Sugimoto (11).
The cerebral infection due to P. penneri described here is, to our knowledge, the first to be described and emphasizes the virulence (20) and the opportunistic character of P. penneri (18). The microorganism invaded the brain from the respiratory tract through a breach of the cranial basis. The interest of the present case is understanding of the emergence of a β-lactamase-hyperproducing mutant of P. penneri during treatment with ceftriaxone. The HugA β-lactamase of P. penneri, sequenced for the first time in the present study, belongs to structural class A of the β-lactamases and was regulated by an equivalent of the amp system, a regulation system of class C β-lactamases (15). The ampR gene encodes a transcriptional regulator, like hugR for P. penneri and cumR for P. vulgaris, whereas ampD encodes an enzyme that controls the induction of the β-lactamase (3, 10). A mutation of the ampD gene results in high-level constitutive expression of the β-lactamase and resistance to broad-spectrum cephalosporins. These mutants can be selected in vivo (5, 15). In the present case, both the β-lactamase and transcriptional regulator genes of the ceftriaxone-susceptible isolate and the ceftriaxone-resistant isolate of P. penneri have similar sequences. This indicates that the ceftriaxone resistance is linked to a mutation elsewhere within the amp system and probably involves a mutation of the equivalent of the ampD gene in P. penneri. Previously, Datz et al. (6) restored the ceftriaxone susceptibility of a ceftriaxone-resistant isolate of P. vulgaris by introducing an ampD gene. In the present case, the selection of a β-lactamase-hyperproducing mutant could be explained by several factors: (i) the cerebral localization of the infection, in which the concentration of ceftriaxone (8) is not sufficient to inhibit the growth of mutants; (ii) the presence of a prosthetic material, which allowed the adherence of microorganisms; and (iii) the large bacterial inoculum, which at the beginning contained a certain proportion of β-lactamase-hyperproducing mutants. The present case emphasizes the necessity to carefully prescribe extended-spectrum cephalosporins as monotherapy for the treatment of severe infections due to P. penneri.
Nucleotide sequence accession number.
The sequence coding for the β-lactamase, named HugA, and a LysR-type regulator, named HugR, can be found in the GenBank database under accession number AF324468.
Acknowledgments
We thank Wladimir Sougakoff for determination of the isoelectric point of the β-lactamase, Reno Frei for the typing of isolates by pulsed-field gel electrophoresis, and Isabelle Jan for technical assistance.
REFERENCES
- 1.Antibiogram Committee of the French Society for Microbioloy. 1998. Statement. Pathol. Biol. 8:I–XVI. [Google Scholar]
- 2.Barthelemy, M., M. Guionie, and R. Labia. 1978. Beta-lactamases: determination of their isoelectric points. Antimicrob. Agents Chemother. 13:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, P. M., and I. Chopra. 1993. Molecular basis of β-lactamase induction in bacteria. Antimicrob. Agents Chemother. 37:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow, J. W., M. J. Fine, D. M. Shlaes, J. P. Quinn, D. C. Hooper, M. P. Johnson, R. Ramphal, M. M. Wagener, D. K. Miyashiro, and V. L. Yu. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585–590. [DOI] [PubMed] [Google Scholar]
- 6.Datz, M., B. Joris, E. A. M. Azab, M. Galleni, J. Van Beeumen, J. M. Frère, and H. H. Martin. 1994. A common system controls the induction of very different genes, the class-A β-lactamase of Proteus vulgaris and the enterobacterial class-C β-lactamase. Eur. J. Biochem. 226:149–157. [DOI] [PubMed] [Google Scholar]
- 7.Grace, M. E., F. J. Gregory, P. P. Hung, and K. P. Fu. 1986. Purification and properties of a β-lactamase from Proteus penneri. J. Antibiot. 39:938–942. [DOI] [PubMed] [Google Scholar]
- 8.Granero, L., M. Santiago, J. Cano, A. Machado, and J. E. Peris. 1995. Analysis of ceftriaxone and ceftazidime distribution in cerebrospinal fluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob. Agents Chemother. 39:2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman, F. W., A. G. Steigerwalt, J. J. Farmer III, and D. J. Brenner. 1982. Identification of Proteus penneri sp. nov., formerly known as Proteus vulgaris indole negative or as Proteus vulgaris biogroup 1. J. Clin. Microbiol. 15:1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honoré, M., M. H. Nicolas, and S. T. Cole. 1989. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol. Microbiol. 3:1121–1130. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro, K., and K. Sugimoto. 1996. Purification and characterization of the Proteus vulgaris BlaA protein, the activator of the β-lactamase gene. J. Biochem. 120:98–103. [DOI] [PubMed] [Google Scholar]
- 12.Joris, B., P. Ledent, O. Dideberg, E. Fonzé, J. Lamotte-Brasseur, J. A. Kelly, J. M. Ghuysen, and J. M. Frère. 1991. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob. Agents Chemother. 35:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajden, S., M. Fuksa, C. Petrea, L. J. Crisp, and J. L. Penner. 1987. Expanded clinical spectrum of infections caused by Proteus penneri. J. Clin. Microbiol. 25:578–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labia, R., J. Andrillon, and F. Le Goffic. 1973. Computerized microacidimetric determination of a β-lactamase Michaelis Menten constants. FEBS Lett. 33:42–44. [DOI] [PubMed] [Google Scholar]
- 15.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miro, E., M. Barthélémy, J. Péduzzi, A. Reynaud, A. Morand, G. Prats, and R. Labia. 1994. Propriétés d’une céphalosporinase de Proteus penneri inhibée par l’acide clavulanique. Pathol. Biol. 42:487–490. [PubMed] [Google Scholar]
- 17.Mohr O’Hara, C., F. W. Brenner, and J. M. Miller. 2000. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 13:534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing; ninth information supplement. M100S9, vol. 19, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Peduzzi, J., A. Reynaud, P. Baron, M. Barthélémy, and R. Labia. 1994. Chromosomally encoded cephalosporinase of Proteus vulgaris Ro104 belongs to Ambler’s class A. Biochim. Biophys. Acta 1207:31–39. [DOI] [PubMed] [Google Scholar]
- 20.Rozalski, A., Z. Sidorczyk, and K. Kotelko. 1997. Potential virulence factors of Proteus bacilli. Microbiol. Mol. Biol. Rev. 61:65–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaki, M., M. Nukaga, and T. Sawai. 1994. Replacement of serine 237 in class A β-lactamase of Proteus vulgaris modifies its unique substrate specificity. Biochemistry 33:1020–1026. [DOI] [PubMed] [Google Scholar]