Abstract
The antistaphylococcal activities of BMS-284756 (T-3811ME), levofloxacin, moxifloxacin, and ciprofloxacin were compared against wild-type and grlA and grlA/gyrA mutant strains of Staphylococcus aureus. BMS-284756 was the most active quinolone tested, with MICs and minimal bactericidal concentrations against S. aureus wild-type strain MT5, grlA mutant MT5224c4, and grlA/gyrA mutant EN8 of 0.03 and 0.06, 0.125 and 0.125, and 4 and 4 μg/ml, respectively. In the time-kill studies, BMS-284756 and levofloxacin exhibited rapid killing against all strains. Ciprofloxacin, however, was not bactericidal for the double mutant, EN8. BMS-284756 and levofloxacin were bactericidal (3 log10 decrease in CFU/ml) against the MT5 and MT5224c4 strains at two and four times the MIC within 2 to 4 h. Against EN8, BMS-284756 was bactericidal within 4 h at two and four times the MIC, and levofloxacin achieved similar results within 4 to 6 h. Both the wild-type strain MT5 and grlA mutant MT5224c4 should be considered susceptible to both BMS-284756 and levofloxacin, and both quinolones are predicted to have clinical efficacy. The in vivo efficacy of BMS-284756, levofloxacin, and moxifloxacin against S. aureus strain ISP794 and its single mutant 2C6(1)-1 directly reflected the in vitro activity: increased MICs correlated with decreased in vivo efficacy. The 50% protective doses of BMS-284756 against wild-type and mutant strains were 2.2 and 1.6 mg/kg of body weight/day, respectively, compared to the levofloxacin values of 16 and 71 mg/kg/day and moxifloxacin values of 4.7 and 61.6 mg/kg/day. BMS-284756 was more potent than levofloxacin and equipotent with moxifloxacin against ISP794 both in vitro and in vivo, while BMS-284756 was more potent than levofloxacin and moxifloxacin against 2C6(1)-1.
The C-6 fluorine of the 7-piperazine-substituted quinolones has been shown to be essential for the enhanced potency of these fluoroquinolones against gram-positive bacteria (4). The quinolone BMS-284756 (T-3811ME), identified by Toyama Chemical Co. (Toyama, Japan), lacks the classical C-6 fluorine of fluoroquinolones and has fluorine incorporated through a C-8 difluoromethyl ether linkage (H. Hayashi, Y. Todo, S. Hamasoto, I. Ojima, M. Yamada, T. Kito, M. Takahata, Y. Watanabe, and H. Narita, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr F-158, 1997). BMS-284756 has demonstrated potent antibacterial activity against staphylococci, streptococci, pneumococci, and anaerobic bacteria compared to other quinolones (8). In addition, this broad-spectrum antibacterial showed efficacy in experimental mouse models of systemic infection and pneumonia (23).
It has been demonstrated that the exposure of bacterial pathogens to fluoroquinolones during antimicrobial therapy can select for bacteria with mutations within the quinolone resistance determining region (QRDR) of the genes encoding target enzymes. The emergence of common pathogens with fluoroquinolone resistance has been documented and presumably leads to clinical failure (1). In this study, the in vitro activity of BMS-284756 against a panel of Staphylococcus aureus strains with resistant genotypes was evaluated. Also, well-characterized S. aureus strains with mutations in the QRDR were employed to examine the bactericidal characteristics of BMS-284756 and comparator quinolones. This evaluation was achieved by using results from MIC and minimum bactericidal concentration (MBC) determinations, time-kill analysis, and murine models of systemic infections.
(The initial report of this work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 September 2000.)
MATERIALS AND METHODS
Chemicals.
BMS-284756 and analogs were obtained from Toyama Chemical Company. Ciprofloxacin was obtained from Bayer Corporation, West Haven, Conn. Levofloxacin was obtained from the manufacturer or was extracted from tablets. Levofloxacin and moxifloxacin were extracted from commercial tablets, purified by recrystallization, and determined to be >99.9% pure by high-performance liquid chromatography analysis.
Strains.
Strains used for time-kill analyses included MT5, MT5224c4, and EN8. MT5 is a genetically defined parental S. aureus strain which was used to select for mutant strains on ciprofloxacin or norfloxacin and has prior mutations in the nov-142 locus (gyrB lle102Ser and Arg144lle) (5). This strain has increased resistance to coumarins but not to fluoroquinolones. MT5224c4 is a single-step grlA mutant (Ser80Phe). EN8 is the result of a genetic cross between two derivatives of S. aureus RN4220 separately containing the two mutations (18). The strains used for in vivo efficacy studies include the parent S. aureus ISP794 and a single mutant, 2C6(1)-1, which were created by stepwise selection on increasing concentrations of ciprofloxacin (3). Strain 2C6(1)-1 contains an Ala116Glu mutation in the GrlA subunit of topoisomerase IV and demonstrates a fourfold increase in the MICs of ciprofloxacin against staphylococci.
MICs and MBCs.
MICs and MBCs were determined using the National Committee for Clinical Laboratory Standards recommended broth microdilution and macrodilution assay, respectively, and were performed in duplicate (16, 17). All tests were carried out in cation-adjusted Mueller Hinton Broth (CAMHB; Becton Dickinson, Cockeysville, Md.). The MIC was defined as the minimum amount of quinolone that resulted in no visible growth after 24 h at 35°C. The MBC was determined according to the minimum number of viable colonies allowed for a 99.9% endpoint as defined by the National Committee for Clinical Laboratory Standards (16).
Time-kill curves.
Rates of killing were determined by measuring the reduction in viable bacteria (log10 CFU/ml) at 0, 1, 2, 4, 6, and 24 h at fixed concentrations of quinolone (14). Experiments were performed in duplicate. If plates contained fewer than 3 × 101 CFU/ml, the number of colonies was considered to be below the limit of quantitation and is provided solely as an estimation (14). Samples of culture containing quinolone were diluted at least 10-fold to minimize drug carryover to the CAMHA plates.
Acute systemic S. aureus infection in mice.
Adult female ICR mice (21 to 23 g; Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) were inoculated intraperitoneally with a sufficient number of pathogens to kill 100% of the untreated animals. Each mouse received ∼2.8 × 108 CFU of ISP794/ml and ∼8 × 107 CFU of 2C6(1)-1 S. aureus strains/ml suspended in 7% sterile hog gastric mucin. Drug was administered at 1 and 5 h after pathogen inoculation. The number of mice that survived in each experimental group was monitored up to 8 days after pathogen inoculation, and the 50% protective doses (PD50s) of the drug-treated animals were determined by the Spearman-Karber nonparametric estimator method (12). Each experimental group consisted of 10 animals, and a minimum of three different concentrations of drug was evaluated per compound. The ranges of doses for BMS-284756, levofloxacin, and moxifloxacin were 10 to 0.625 mg/kg of body weight/day, 25 to 1.56 mg/kg/day, and 25 to 1.56 mg/kg/day, respectively, against the wild-type ISP794 and were 10 to 0.625 mg/kg/day, 100 to 25 mg/kg/day, and 50 to 12.5 mg/kg/day, respectively, against the mutant strain. The statistical comparison of PD50s was carried out using the Z test as described by Hubert (12).
RESULTS
The in vitro activities of BMS-284756, ciprofloxacin, moxifloxacin, and levofloxacin were initially investigated against a panel of S. aureus strains with various QRDR mutations (Table 1). The GyrA Ser84Leu mutation in SS1 (2) increased MICs fourfold for BMS-284756 compared to the MICs against MT5 while single mutations in GrlA in two of three strains did not. Against EN14 with a combination of the GyrA Ser84Leu mutation and a GrlB Asn470Asp mutation, the MICs increased 32-fold for BMS-284756 but remained below 4 μg/ml. MICs of levofloxacin and ciprofloxacin for EN14 also increased to 4 μg/ml. BMS-284756 was as susceptible to efflux as ciprofloxacin, as both quinolones resulted in a fourfold increase in MICs against a NorA overexpressor strain, MT23142, compared to that against the parent strain ISP794.
TABLE 1.
MICs of BMS-284756, ciprofloxacin, moxifloxacin, and levofloxacin against mutant and wild-type strains of S. aureus
| S. aureus straina | Source or reference | Genotype | MIC or MIC/MBC (μg/ml)b
|
|||
|---|---|---|---|---|---|---|
| BMS-284756 | LVX | MXF | CIP | |||
| ISP794 | 22 | Wild-type parent strain 8325 | 0.015 | 0.125 | 0.06 | 0.25 |
| MT5 | 5 | gyrB (lle 102Ser and Arg144lle) | 0.03/0.06 | 0.25/0.25 | 0.125/0.25 | 0.5/0.5 |
| MT111 | 18 | grlA548 (Ala116Glu) | 0.03 | 0.5 | 0.25 | 1 |
| MT52222 | 18 | grlA541 (Ser80Phe) | 0.03 | 0.5 | 0.25 | 1 |
| MT5224c4 | 18, 24 | grlA542 (Ser80Phe) | 0.125/0.125 | 1/1 | 0.25/0.5 | 2/2 |
| MT5224c9 | 18, 24 | grlB543 (Asn470Asp) | 0.06 | 0.5 | 0.125 | 1 |
| MT23142 | 19 | NorA overexpressor | 0.06 | 0.25 | 0.03 | 0.5 |
| SS1 | 2 | gyrA (Ser84Leu) | 0.125 | 0.25 | 0.125 | 0.25 |
| RN4220 | 15 | Wild-type parent strain 8325-4 r- | 0.06/0.125 | 0.5/05 | 0.125/0.25 | 1/1 |
| EN20 | 18 | grlA542 (Ser80Phe) | 0.03 | 0.25 | 0.25 | 1 |
| EN22 | 18 | grlB543 (Asn470Asp) | 0.03 | 0.5 | 0.25 | 1 |
| EN14 | 2 | grlB543 (Asn470Asp)/gyrA (Ser84Leu) | 1 | 4 | 1 | 4 |
| EN8 | 18 | grlA542 (Ser80Phe)/gyrA (Ser84Leu) | 4/4 | 16/16 | 4/4 | 64/>64 |
| QC | ATTC 29213 | Wild type | 0.03 | 0.25 | 0.03 | 0.25 |
Mutants MT5, MT111, MT52222, MT5224c4, MT5224c9, MT23142, and SS1 were derived from ISP794. Mutants EN20, EN22, EN14, and EN8 were derived from RN4220. QC, quality control.
LVX, levofloxacin; MXF, moxifloxacin; CIP, ciprofloxacin.
The MICs of BMS-284756 against parental MT5, MT5224c4, and EN8 were 16-fold lower than those of ciprofloxacin, 4- to 8-fold lower than those of levofloxacin, and 1- to 4-fold lower than those of moxifloxacin (Table 2). The MBCs of all quinolones against parental MT5 were equivalent or twofold higher than their corresponding MICs (Table 1). The MBCs of BMS-284756, levofloxacin, and moxifloxacin for the single and double mutants were also equivalent to their MICs, suggesting that mutations in the target genes do not change the bactericidal character of these quinolones. The MICs of BMS-284756 against the wild-type ISP794 and the Ala116Glu mutant, 2C6(1)-1, were similar, while the MICs of moxifloxacin, levofloxacin, and ciprofloxacin were increased 4-, 8-, and 32-fold, respectively (Table 2).
TABLE 2.
Comparison of in vitro and in vivo activities of BMS-284756, levofloxacin, and moxifloxacin against wild-type and single mutant strains of S. aureus
| Strain | Reference | Genotype | MIC (μg/ml)a
|
PD50 (95% CL)a (mg/kg)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| BMS-284756 | LVX | MXF | CIP | BMS-284756 | LVX | MXF | |||
| ISP794 | 22 | WTb | 0.015 | 0.125 | 0.06 | 0.125 | 2.2 (1.05–4.5) | 16 (11.4–23.9) | 4.7 (2.78–8.05) |
| 2C6(1)-1 | 3 | GrlA Ala116Glu | 0.03 | 1 | 0.25 | 4 | 1.6 (0.88–3.09) | 61.6 (58.1–86.0) | 16.5 (13.09–20.78) |
CL, confidence limits for the log PD50 were calculated by using the normal distribution.
WT, wild type.
LVX, levofloxacin; MXF, moxifloxacin; CIP, ciprofloxacin.
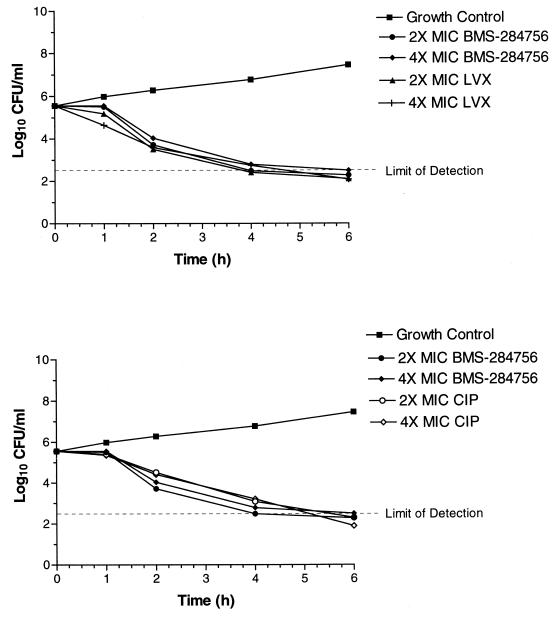
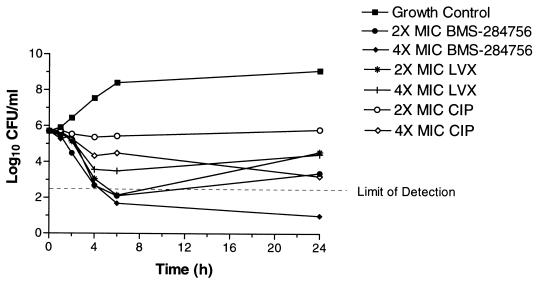
Three quinolones, BMS-284756, levofloxacin, and ciprofloxacin, were further characterized in the time-kill studies and generally exhibited rapid killing against all strains. The only exception was for ciprofloxacin, which did not exhibit rapid killing against EN8. BMS-284756 and levofloxacin were bactericidal (≥3 log10 decrease in CFU/milliliter) against the MT5 strain within 4 h (data not shown). Against MT5224c4 at two and four times the MIC, BMS-284756 and ciprofloxacin showed a similar degree of killing, with a bactericidal effect within 4 to 6 h. Levofloxacin also demonstrated bactericidal activity against this strain at all concentrations tested within 4 h. Against EN8, BMS-284756 was bactericidal within 4 h at two and four times the MIC, and levofloxacin achieved similar results within 4 to 6 h (Fig. 1). At two and four times the MIC, ciprofloxacin did not achieve a 3 log10 decrease in CFU/milliliter after 6 h against the double-mutant EN8 (Fig. 2). As shown in Fig. 2, at a concentration of four times the MIC, BMS-284756 was the only quinolone that maintained killing up to 24 h compared to ciprofloxacin and levofloxacin.
FIG. 1.
Time-kill analysis of BMS-284756, levofloxacin (LVX), and ciprofloxacin (CIP) against S. aureus MT5224c4 GrlA at two and four times the MIC for 6 h.
FIG. 2.
Time-kill analysis of BMS-284756, levofloxacin (LVX), and ciprofloxacin (CIP) against S. aureus EN8 GrlA/GyrA at four times the MIC for 24 h.
In general, at equivalent multiples of the MIC, levofloxacin and ciprofloxacin had a greater degree of killing (ΔCFU/milliliter) than BMS-284756 against MT5 and MT5224c4 strains at 1 or 2 h, although by 4 h, all quinolones demonstrated a similar degree of killing (Fig. 1 and 2). In terms of concentration, against MT5 and MT5224c4 strains, BMS-284756 exhibited bactericidal (3 log10 decrease in CFU/milliliter) activity in 4 h at 0.06 μg/ml (two times the MIC) while levofloxacin was bactericidal at 4 h at 0.5 μg/ml (two times the MIC). In general, BMS-284756 was equally bactericidal compared to levofloxacin and ciprofloxacin against these strains at an 8 times lower concentration and 16 to 64 times lower concentrations, respectively.
The relevance of in vitro bactericidal activity of quinolones to their in vivo efficacy was determined by a comparison of efficacy against the wild type and a mutant strain in an animal model. Upon testing the parental MT5 in vivo, the resulting PD50s of levofloxacin were unusually high compared to published values for other wild-type strains of S. aureus (6, 13). The PD50s of the MT5224c4 and EN8 mutants, therefore, were irregular or could not be calculated from the high concentrations of quinolone tested. These results might be explained by the attenuated virulence of these strains that required a high inoculum to achieve a lethal dose. However, another wild-type strain, ISP794, resulted in expected PD50s, and therefore, we chose to test ISP794 and its mutant, 2C6(1)-1, for the relative efficacy of the quinolones. The MICs of BMS-284756 against the wild-type ISP794 and a single mutant, 2C6(1)-1, were 0.015 and 0.03 μg/ml, respectively, and for levofloxacin the MICs were 0.125 and 1 μg/ml, respectively (Table 2). MICs of moxifloxacin against these strains were comparable to those of levofloxacin. The PD50 of BMS-284756 was not affected by the single mutation, while the PD50 of levofloxacin against the mutant 2C6(1)-1 was greater compared to the wild-type value by a factor of 4.4 (P < 0.0001). The PD50 of moxifloxacin also increased significantly for the 2C6(1)-1 mutant compared to the wild type (P < 0.0001).
DISCUSSION
BMS-284756 was more active in vitro than levofloxacin, moxifloxacin, and ciprofloxacin against a variety of S. aureus strains with various topoisomerase mutations. The MICs of BMS-284756 remained at or below 4 μg/ml for all strains, including those containing single and double mutations of gyrA, grlA, and/or gyrB. The fact that MICs of BMS-284756 were increased for GyrA single mutations but not GrlA mutations in two of three strains suggests that distinct from other newer quinolones, which target topoisomerase IV in S. aureus, DNA gyrase is the primary target of BMS-284756 in S. aureus (7, 10, 20). Although other newer quinolones have primarily targeted DNA gyrase in Streptococcus pneumoniae (7, 10, 20), all previously tested quinolones have had topoisomerase IV as their primary target in S. aureus (18). Further evidence for DNA gyrase as the most sensitive target in gram-positive bacteria for BMS-284756 includes initial selection of this enzyme in resistance studies (3, 9).
Mutations in the genes encoding subunits of either topoisomerase (DNA gyrase or topoisomerase IV) did not affect the bactericidal activity of BMS-284756 or the other quinolones, when MBCs are adjusted for MIC levels. BMS-284756 achieved rapid time-kill kinetics with similar results to comparators at concentrations 8 to 64 times lower. These data are not unexpected, as other investigators have shown that although ciprofloxacin is bacteriostatic against strains with double mutations, newer quinolones remain bactericidal (18).
The in vivo efficacy of BMS-284756, moxifloxacin, and levofloxacin against the S. aureus parent strain ISP794 and single-mutation strain 2C6(1)-1 directly reflected the in vitro activity. BMS-284756 was more potent than levofloxacin both in vitro and in vivo against both strains and was more potent than moxifloxacin against the mutant strain. The enhanced in vivo potency of BMS-284756 compared to other quinolones demonstrated in this study confirms the data of Takahata et al. (23), which showed the efficacies of BMS-284756 against a single GrlA mutant of S. aureus were 30.3- and 70.4-fold greater than those of levofloxacin and ciprofloxacin, respectively. Against a double GyrA and GrlA mutant, BMS-284756 was also more potent than ciprofloxacin and levofloxacin (23).
Although a formal mathematical correlation between protein binding and clinical efficacy is not clearly understood, protein binding has become an important parameter in dose selection for quinolone antibiotics. Assuming that only free drug is pharmacologically active, quinolones may require higher doses to achieve free drug exposures consistent with pharmacodynamic targets (11). Protein binding was not assessed in this study; however, an ex vivo determination of protein binding of BMS-284756 was performed in a clinical study following 28 days of oral dosing with 400 mg of BMS-284756. The protein binding was determined to be 75% and was concentration and time independent (A. Bello, D. Hollenbaugh, D. A. Gajjar, L. Christopher, and D. M. Grasela, Abstr. 41st ICAAC, abstr. A-45, 2001). The free area under the concentration-time curves of BMS-284756 at 600- and 400-mg doses were 29.5 and 19.8 μg · h/ml (D. A. Gajjar, R. Russo, A. Bello, L. Christopher, M. Geraldes, and D. M. Grasela, Abstr. 41st ICAAC, abstr. A-44, 2001; C. Stewart, D. A. Gajjar, A. Bello, L. Christopher, D. Hollenbaugh, Z. Ge, and D. M. Grasela, Abstr. 41st ICAAC, abstr. A-46, 2001), respectively, which would result in free area under the concentration-time curve-to MIC ratios of 983 and 660, respectively, for the single mutant 2C6(1)-1. The maximum concentrations of drug in serum were 10.2 and 7.22 μg/ml, respectively (21). It is evident from the ratios that the free-drug exposures achieved are adequate and consistent with pharmacodynamic targets. The range of MICs of BMS-284756 against S. aureus strains with characterized topoisomerase mutations obtained in this study was 0.03 to 4 μg/ml (n = 11). MICs of BMS-284756 were obtained by Takahata et al. (n = 6), with a range of 0.063 to 1.56 μg/ml against S. aureus strains with similar topoisomerase mutations. These data, although limited in the number of strains, suggest that there is sufficient free-drug concentration of BMS-284756 at a therapeutically targeted dose to afford coverage of certain resistant strains in the clinic. Future resistance studies will support the identification of DNA gyrase as the primary target of BMS-284756 and further evaluate its activity against quinolone-resistant bacteria.
Acknowledgments
We thank Kwasi Ohemeng, Van Nguyen, and Hong Huang for assistance in the extraction and purification of quinolones.
REFERENCES
- 1.Ball, P. 1994. Bacterial resistance to fluoroquinolones: lessons to be learned. Infection 22:S140–S147. [DOI] [PubMed] [Google Scholar]
- 2.Bisognano, C., P. E. Vaudaux, D. P. Lew, E. Y. W. Ng, and D. C. Hooper. 1997. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 41:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Discotto, L. F., L. E. Lawrence, K. L. Denbleyker, and J. F. Barrett. 2001. Staphylococcus aureus mutants selected by BMS-284756. Antimicrob. Agents Chemother. 45:3273–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domagala, J. 1994. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 33:685–706. [DOI] [PubMed] [Google Scholar]
- 5.Fournier, B., and D. C. Hooper. 1998. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob. Agents Chemother. 42:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu, K. P., S. C. Lafredo, B. Foleno, D. M. Isaacson, J. F. Barrett, A. J. Tobia, and M. E. Rosenthale. 1992. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob. Agents Chemother. 36:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda, H., and K. Hiramatsu. 1999. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huzcko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-F(6)-quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartman-Neumann, S., K. DenBleyker, L. A. Pelosi, L. E. Lawrence, J. F. Barrett, and T. J. Dougherty. 2001. Selection and genetic characterization of Streptococcus pneumoniae mutants resistant to the Des-F(6) quinolone BMS-284756. Antimicrob. Agents Chemother. 45:2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton, V. J., J. E. Ambler, and L. M. Fisher. 2000. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitro. Antimicrob. Agents Chemother. 44:3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houston, A. K., and R. N. Jones. 1994. Postantibiotic effect of DU-6859a and levofloxacin as compared with ofloxacin. Diagn. Microbiol. Infect. Dis. 18:57–59. [DOI] [PubMed] [Google Scholar]
- 12.Hubert, J. J. 1992. Bioassay, p.73. Kendall/Hunt Publishing Co., Dubuque, Iowa.
- 13.Klesel, N., K. H. Geweniger, P. Koletzki, D. Isert, M. Limbert, A. Markus, G. Riess, H. Schramm, and P. Iyer. 1995. Chemotherapeutic activity of levofloxacin (HR 355, DR-3355) against systemic and localized infections in laboratory animals. J. Antimicrob. Chemother. 35:805–819. [DOI] [PubMed] [Google Scholar]
- 14.Knapp, C., and J. A. Moody. 1992. Tests to assess bactericidal activity, p.5.16.1–5.16.20. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, D.C.
- 15.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O’Reilly, P. M. Sclievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 305:709–712. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Document M26-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7–A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Ng, E. Y., M. Trucksis, and D. C. Hooper. 1996. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng, E. Y. W., M. Trucksis, and D. C. Hooper. 1994. Quinolone resistance mediated by norA: physiological characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob. Agents Chemother. 38:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan, X. S., and L. M. Fisher. 1997. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob. Agents Chemother. 41:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan, B. M., C. E. Mazzucco, L. E. Lawrence, H. Ho, G. Warr, J. F. Barrett, and M. Frosco. A comparison of the bactericidal activities and post-antibiotic effects of the des-F(6)-quinolone BMS-284756, levofloxacin, and ciprofloxacin against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 22.Stahl, M. L., and P. A. Pattee. 1983. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J. Bacteriol. 154:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahata, M., J. Mistuyama, Y. Yamashiro, M. Yonezawa, H. Aaraki, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 1999. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob. Agents Chemother. 43:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trucksis, M., J. S. Wolfson, and D. C. Hooper. 1991. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J. Bacteriol. 173:5854–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]