Abstract
Topically applied microbicides that eradicate pathogens at the time of initial exposure represent a powerful strategy for the prevention of sexually transmitted infections. To aid in the further development of an effective topical microbicide, we assessed the minimum cidal concentration (MCC) of two cecropin peptides, D2A21 and D4E1, and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis in vitro. The MCC of peptide D2A21was 5 μM (18.32 μg/ml), and that of peptide D4E1 was 7.5 μM (21.69 μg/ml). The MCC of gel formulations containing 2% D2A21 was 0.2 mM (0.7 mg/ml), and that of gel formulations containing 0.5% D2A21 was 0.2 mM (0.7 mg/ml). There was no significant variation in the results when two different C. trachomatis strains were tested, and the addition of 10% human blood did not significantly alter the MCCs. pH values above and below 7 reduced the activity of the D2A21 peptide alone, but the MCC of the 2% D2A21 gel formulation was only slightly altered at the various pHs tested. Ultrastructural studies indicated that C. trachomatis membranes were disrupted after D2A21 exposure, resulting in leakage of the cytoplasmic contents. These in vitro results suggest that these cecropin peptides may be an effective topical microbicide against C. trachomatis and support the need for further evaluation.
Topical microbicides that can be self-administered prior to sexual contact without the need for acquiescence by the sexual partner and that are broadly active against sexually transmitted infection (STI)-causing pathogens represent a powerful strategy for the prevention and a reduction in the spread of STIs such as those caused by Chlamydia trachomatis. Even though chlamydial infections can be treated with antibiotics, many infections are undetected. It is estimated that approximately 75% of chlamydial infections in women and 50% in men are asymptomatic and therefore are undetected and untreated (13). Asymptomatic infections can be spread unknowingly to sexual partners (7, 20, 27), and untreated chlamydial infections can lead to serious reproductive sequelae, particularly pelvic inflammatory disease, infertility, and ectopic pregnancy in women. The four million new cases a year caused by C. trachomatis make it the most commonly reported bacterial infectious disease in the United States (5, 15).
Chlamydiae are obligate intracellular bacteria that have a unique biphasic developmental cycle. Elementary bodies (EBs) are the infectious but nonmetabolically active chlamydial form adapted for extracellular survival. The EBs attach to and are phagocytosed by a eukaryotic host cell, where they reorganize into noninfectious but metabolically active reticulate bodies inside a membrane-bound vacuole called an inclusion. The reticulate bodies divide by binary fission asynchronously and, after 30 to 48 h, recondense to form EBs. At this stage, the host cell lyses and the EBs are released and can infect new eukaryotic cells or be spread in genital secretions to a sexual partner (30). Due to this unique developmental cycle, an ideal antichlamydial topical microbicide should be active against the extracellular, infectious chlamydial EBs in order to prevent infection.
The currently available spermicidal products containing detergents all have significant limitations as topical microbicides. For example, nonoxynol-9 (the most common active ingredient in currently available commercial spermicidal products) has been shown to irritate the vaginal and rectal epithelia upon repeated use, is active against normal flora, and has been shown to have little or no effect on human immunodeficiency virus, C. trachomatis, or Neisseria gonorrhoeae in recent clinical studies (9, 28, 32). An ideal topical microbicide should not only kill STI-causing pathogens and be potentially spermicidal but also not disrupt the normal flora of the vagina or rectum and not cause cytotoxicity to the vaginal or rectal epithelium.
Cecropin peptides are a group of antibacterial, cationic peptides that were originally identified in the pupae of the cecropia moth and have recently been identified in other insects (e.g., bactericidin, moricin, and sarcotoxin) as well as in pig intestines (cecropin P) and tunicates (4). They form amphipathic alpha-helices and lack hemolytic activities (12, 26). It has been suggested that the mode of action for this class of peptides is the creation of pores or channels across the bacterial membrane (1, 26). Several cecropins have been shown to cause a disruption of the integrity of the lipid bilayer, but other peptides within this class have also been shown to release mitochondrial respiratory control, inhibit protein import, and, at higher concentrations, inhibit respiration in a variety of bacteria (4). Cecropin peptides have demonstrated strong activities against gram-negative and gram-positive bacteria as well as fungi and viruses (10, 22, 33). Cecropins also have minimal cytotoxic activities against mammalian cells, making them ideal candidates for topical application (1, 4). To determine if the peptides D2A21 and D4E1 could function as effective topical microbicides, we examined the in vitro activities of these two cationic antimicrobial peptides and four gel formulations containing 2%, 0.5%, 0.1%, and 0% (placebo) D2A21 against C. trachomatis.
MATERIALS AND METHODS
Cell culture.
Low-passage (<15 passages) McCoy mouse fibroblast cells (ATCC CRL 1696) were maintained in antibiotic-free Eagle’s minimal essential medium with 10% fetal calf serum (EMEM). The McCoy cells were checked once per month for Mycoplasma contamination.
Bacterial strains.
Two urogenital strains of C. trachomatis, serovars D (UW-3/Cx) and F (UW-6/Cx), were grown in McCoy mouse fibroblast cells in antibiotic-free EMEM, purified on Renografin density gradients, and stored at −70°C in SPG (219 mM sucrose, 3.8 mM KH2PO4, 8.6 mM Na2HPO4, 4.9 mM l-glutamic acid). Immediately prior to use, the purified organisms were thawed and diluted in SPG. The reference Escherichia coli strain (ATCC 25922) was stored frozen at −70°C in 50% Trypticase soy broth-50% fetal calf serum. Twenty-four hours prior to use, the organism was plated on a Luria-Bertani (LB) agar plate and incubated overnight at 35°C.
Peptides.
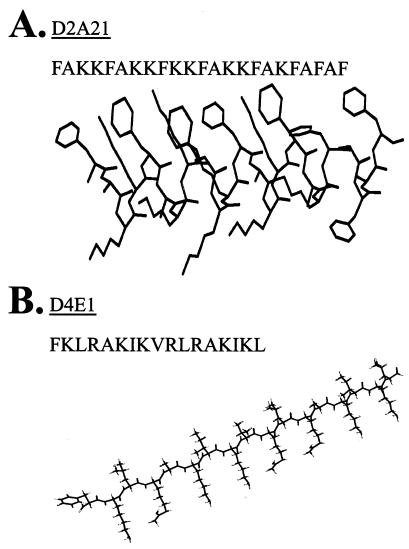
The amino acid sequences and structures of the peptides D2A21 and D4E1 are shown (Fig. 1). Lyophilized D2A21 and D4E1 were provided by Demegen, Inc. (Pittsburgh, Pa.), rehydrated in sterile H2O at a 1 mM concentration, and kept frozen in single aliquots at −70°C until use. Acetate and sodium counterions were considered in calculating these concentrations. Gel formulations were provided by Demegen, Inc., and consisted of 3.25% hydroxyethylcellulose NF, 0.9% (wt/vol) sodium chloride, and either 0% (placebo gel), 0.1%, 0.5%, or 2% D2A21 in water at pH 5.3. The peptide was tested for stability in the gel formulations by high-performance liquid chromatography and found to be stable for up to 3 months. Gel formulations were stored at 4°C until use. Serial dilutions of the peptides or gel formulations were made in SPG. After each dilution, the peptide or gel formulation was vortexed for 15 to 30 s and was evenly distributed. All dilutions were made fresh the day of the experiment.
FIG. 1.
The amino acid sequences and structures of the peptides D2A21 and D4E1. (A) The amphipathic nature of the D2A21 peptide is shown with the hydrophobic amino acids on the top of the molecule. The peptide is assumed to be in an alpha-helical conformation when it is near or in the membrane. The hydrogens are not shown. (B) The D4E1 peptide is depicted in an amphipathic beta-sheet conformation. The nonpolar face is on the top, while the polar charged face is below the plane of the molecule.
Antibiotic controls.
Polymyxin B sulfate and penicillin G (Sigma Chemical Co., St. Louis, Mo.), at initial concentrations of 1.4 mM (2 mg/ml) and 5.4 mM (2 mg/ml), were used as positive and negative controls, respectively, in each of the preinoculation minimum cidal concentration (MCC) assays. EMEM and phosphate-buffered saline were used as controls in each of the AlamarBlue cytotoxicity assays. Tetracycline at an initial concentration of 4.1 mM (2 mg/ml; Sigma) was used as the positive control in the E. coli MCC assay. All controls and their dilutions were prepared fresh the day of the assay and were run in parallel with each assay.
AlamarBlue cytotoxicity assays.
To assess McCoy cell toxicity caused by the peptides or gel formulations containing D2A21 in the C. trachomatis assay, the preinoculation MCC assays (standard MCC and modified ultrafiltration MCC methods) were duplicated except that no C. trachomatis organisms were added. For the standard MCC method, cytotoxicity to the McCoy cells by the peptides or the gel formulations was determined as previously described (17, 18). For the modified ultrafiltration MCC method, serial dilutions of the peptides or gel formulations were made in SPG. The dilutions were filtered in Centricon-30 microconcentrators (Amicon, Beverly, Mass.) per the manufacturer’s instructions for 30 min at 4°C. The retenate was diluted 1:40 in SPG, aliquots were added to 24-h monolayers of McCoy cells, and the cells were treated as previously described (8, 17, 18, 23). Each assay was performed in triplicate, and blank wells with EMEM only and no cells were used to blank each microtiter plate.
Preinoculation MCC assays.
In order to assess the topical killing action of these peptides, the C. trachomatis EBs were exposed to the peptides or gel formulations prior to inoculation. Based on the results from the AlamarBlue cytotoxicity assay, either the previously described standard MCC method or the modified ultrafiltration MCC method was used to assess the antichlamydial activity of the peptide or gel formulation (17, 18, 36). The initial concentrations of the peptide solutions after dilution in SPG and addition of the organism were 0.1 mM (0.37 mg/ml) for D2A21 and 0.06 mM (0.17 mg/ml) for D4E1. The concentrations of D2A21 in the gel formulations were 2% (5.9 mM; 21.6 mg/ml), 0.5% (1.5 mM; 5.5 mg/ml), 0.1% (0.3 mM; 1.1 mg/ml), and 0% (0 mM). The 2% gel formulation containing D2A21 was initially diluted 1:4 in SPG prior to addition of the organism. The 0.5 and 0.1% gel formulations containing D2A21 were initially diluted 1:2 in SPG prior to addition of the organism. C. trachomatis EBs in SPG were then added to serial dilutions of the peptides or gel and vortexed, resulting in even distribution of the mixtures. The lowest concentration of peptide or gel formulation that showed 100% killing was defined as the MCC. All assays were performed in triplicate, three fields per well were counted, and the inclusion counts were averaged. The polymyxin B positive control, penicillin G negative control, and placebo gel containing no D2A21 were run in parallel each time the assay was performed. Each time point included two additional controls. The first was an organism control in which the C. trachomatis EBs with no drug were incubated and diluted following either the standard MCC method or the modified ultrafiltration MCC method to monitor normal inclusion formation. The second was a cell control in which SPG only (no drug and no organism) was incubated and diluted following either the standard MCC or the modified ultrafiltration MCC method in order to monitor normal McCoy cell morphology.
Modified ultrafiltration MCC.
The modified ultrafiltration MCC method was used to assess the antichlamydial activities of the gel formulations containing D2A21 that could not be assessed using the standard MCC method due to cytotoxicity to the McCoy cells in our C. trachomatis assay. C. trachomatis serovar D or F (107 inclusion-forming units [IFU]) in SPG were added to equal volumes of gel formulation containing D2A21 dilutions in SPG. Dilutions of polymyxin B positive and penicillin G negative control antibiotic solutions and placebo gel dilutions containing no D2A21 were run in parallel in each assay. Aliquots were removed immediately after addition of the organism (time, 5 min) and after 120 min of exposure and were filtered in a Centricon-30 microconcentrator for 30 min at 4°C according to the manufacturer’s instructions. The retentate was diluted 1:40 in SPG, aliquots were added to 24-h McCoy cell monolayers in 96-well microtiter plates and centrifuged for 1 h, any unbound organisms and residual peptide mixture were removed, and the infected cell monolayers were overlaid with antibiotic-free EMEM containing 1 μg of cycloheximide/ml (EMEM-C). The cultures were incubated for 48 h, and the cells were fixed and stained as described previously (17, 18).
Preinoculation MCC assay in the presence of 10% human blood.
The effect of human blood was examined using the same preinoculation MCC assays described above, with the exception that 10% human blood at pH 7.0 was added to the peptide, gel formulations containing D2A21, the placebo gel containing no D2A21, and the organism and cell controls as previously described (17, 18).
Preinoculation MCC assays with pH alterations.
The effect of different pH values on the MCCs using the same preinoculation MCC assays described above was examined, except that the peptide, gel formulations containing D2A21, and the placebo gel containing no D2A21 dilutions, as well as the organism and cell control solutions, were adjusted to pH 4, 5, 6, 7, or 8 with 1 M Na2HPO4, 1 M KH2PO4, or 1 M KCl prior to adding the organisms as previously described (17, 18). The percent killing at each of the pH values was derived by comparison with an organism control at that same pH.
Postinoculation MIC assay.
MIC assays to determine if D2A21 could penetrate eukaryotic cells and inhibit chlamydial inclusion formation in previously infected cells were determined as described (29). In brief, McCoy cell monolayers in 96-well microtiter plates were inoculated with 105 IFU of C. trachomatis serovar D and centrifuged for 1 h, unbound organisms were aspirated, and the infected cell monolayers were overlaid with 10-fold serial dilutions of D2A21 in EMEM-C starting at 0.1 mM or 0.37 mg/ml. The cultures were incubated for 48 h, and the cells were fixed and stained (29). A tetracycline positive control and penicillin G negative control were run in parallel each time the assay was performed. An organism control (no drug added to overlay medium) and a cell control (uninfected McCoy cells with no D2A21 in overlay medium) were included each time the assay was performed. All assays were performed in triplicate, three fields per well were counted, and the inclusion counts were averaged.
E. coli MCC assay.
For comparison purposes, the D2A21 MCCs were determined with a reference E. coli laboratory strain by using methods described previously (10). In brief, E. coli ATCC 25922 was grown at 37°C in LB broth. Aliquots in log phase (optical density at 600 nm [OD600] ≈ 0.4 to 0.6) were resuspended in serial dilutions of either the peptide D2A21 (initial concentration, 0.1 mM [0.37 mg/ml]) or tetracycline (initial concentration, 4.1 mM [2 mg/ml]) in fresh LB broth at approximately 106 CFU/ml and exposed for 2 and 18 h with aeration at room temperature. After these times, samples were pelleted, diluted serially, and plated on LB agar plates to obtain counts of viable cells. These experiments were performed in triplicate on separate days, and the results were averaged. The MCC was taken as the lowest concentration of drug that resulted in 100% reduction of colony formation after exposure to and removal of the test agent.
Electron microscopy of C. trachomatis exposed to the D2A21 peptide.
D2A21 (5 μM; 18.3 μg/ml) was incubated with C. trachomatis EBs for 90 min. The treated organisms were prepared as described previously (17) for embedding and thin sectioning for examination in a Phillips model CM-10 transmission electron microscope. Organisms incubated in SPG were treated as described above and examined for typical morphology.
Statistical analysis.
All assays were performed in triplicate, and the results were averaged. All data are shown as the mean ± standard deviation.
RESULTS
AlamarBlue cytotoxicity assay.
Under the conditions of the standard MCC assay, only dilutions of the gel formulation containing 2% D2A21 (5.9 mM [21.6 mg/ml]) showed significant cytotoxicity to the McCoy mouse fibroblast cells (Table 1). The MCC assay was modified, incorporating an ultrafiltration step to remove peptides from the chlamydial EBs after exposure. The McCoy cell toxicity of the 2% gel formulation containing D2A21 was eliminated by using this modified ultrafiltration MCC assay (Table 1). The MCC of the 2% gel formulation as well as the placebo containing no D2A21 was determined using the modified ultrafiltration method. Under the conditions of the standard MCC assay, neither the peptides D2A21 or D4E1 (data not shown), dilutions of the gel formulation containing 0.5 or 0.1% D2A21, nor the placebo containing 0% D2A21 (Table 1) showed significant toxicity to the McCoy mouse fibroblast cells and were thus tested using the standard MCC protocol.
TABLE 1.
Cytotoxic effects of gel formulations containing 0 to 2% D2A21 in AlamarBlue cytotoxicity assay
| Gel formulationa (% D2A21) | MCC methodb | CCc
|
% Cytotoxicity at 1:8 dilutiond | |
|---|---|---|---|---|
| 50% | 90% | |||
| 2 | Standard | 1:512 (11.5 μM [42 μg/ml]) | 1:8 (0.8 mM [2.7 mg/ml]) | 93.9 |
| 2 | Mod. ultrafiltration | Neg | Neg | 8.2 |
| 0.5 | Standard | Neg | Neg | 0 |
| 0.1 | Standard | Neg | Neg | 0 |
| 0 | Standard | Neg | Neg | 0 |
| 0 | Mod. ultrafiltration | Neg | Neg | 0 |
Values represent the concentrations of D2A21 in the gel after addition of an equal volume of SPG. 0%, placebo.
Standard, standard preinoculation MCC method was used; Mod. ultrafiltration, modified MCC method with ultrafiltration was used as described in Materials and Methods.
Cytotoxic concentration (CC) is the dilution (concentration in parentheses) that caused the indicated percent cytotoxicity to McCoy mouse fibroblast cells. Neg, no concentration tested achieved a 50 or 90% CC.
% Cytotoxicity = [(mean OD570 − mean OD600 of no drug control) − (mean OD570 − mean OD600 of test)/(mean OD570 − mean OD600 of no drug control)] × 100. Assays were performed in triplicate, and results were averaged.
Preinoculation MCC assays.
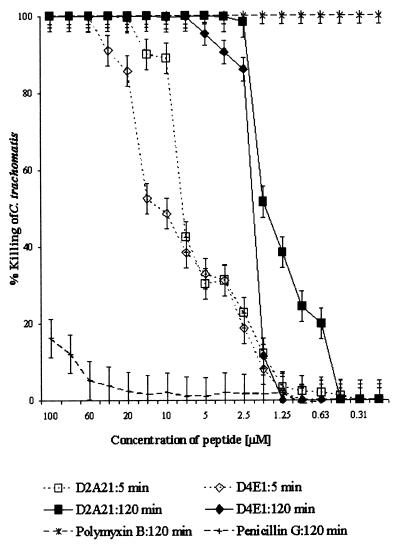
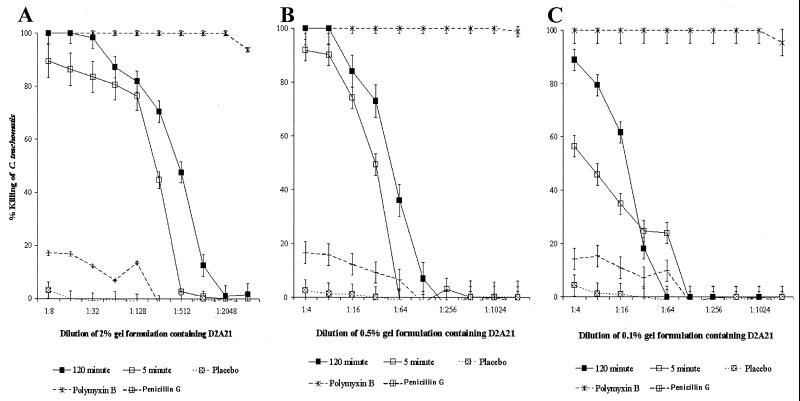
Both D2A21 and D4E1 killed chlamydial EBs immediately after exposure (5 min) at 20 μM (73.3 μg/ml) and 60 μM (173 μg/ml), respectively (Fig. 2). The peptides D2A21 and D4E1 achieved an MCC (100% killing) at 5 μM (18.3 μg/ml) and 7.5 μM (21.7 μg/ml), respectively, after 120 min of exposure. A 32-fold dilution (0.2 mM [0.7 mg/ml]) of the gel formulation containing 2% D2A21 achieved complete killing after 120 min of exposure and killed 90% of the chlamydial organisms immediately (time, 5 min) at a 1:8 dilution (0.7 mM [2.7 mg/ml]) (Fig. 3A). The gel formulation containing 0.5% D2A21 achieved an MCC at an eightfold dilution (0.2 mM [0.7 mg/ml]) after 90 min of exposure and an MCC at a fourfold dilution (0.4 mM [1.4 mg/ml]) after only 30 min of exposure (Fig. 3B). The gel formulation containing 0.1% D2A21 did not achieve complete killing at any of the dilutions tested against C. trachomatis (Fig. 3C). The placebo gel containing 0% D2A21 had no activity (Table 2). Peptides D2A21 and D4E1 demonstrated no significant variation in MCC value between the two strains tested (Table 3).
FIG. 2.
Comparison of the antichlamydial activities of serial dilutions of antimicrobial peptides D2A21 and D4E1. The MCC preinoculation assay was used, and the percent killing of C. trachomatis serovar D after 5 and 120 min of exposure to each peptide dilution or the controls was plotted. The initial concentrations of polymyxin B and penicillin G were 1.4 mM (2 mg/ml) and 5.4 mM (2 mg/ml), respectively. Values were calculated in comparison to that of the organism-only control according to the following formula: (mean IFU of organism control − mean IFU of test)/mean IFU of organism control × 100. The standard deviations from triplicate tests are indicated by error bars. Results for the polymyxin B positive and penicillin G negative controls are also shown.
FIG. 3.
Activities of the gel formulations containing 2% D2A21 against C. trachomatis serovar D in the modified ultrafiltration MCC assay (A), 0.5% D2A21 in the standard MCC assay (B), or 0.1% D2A21 in the standard MCC assay (C). The percent killing of the D2A21-containing gel formulation after 5 and 120 min in the MCC preinoculation assay is plotted, and standard deviations from triplicate tests are indicated by error bars. Values were calculated in comparison to that of an organism-only control according to the following formula: (mean IFU of organism control − mean IFU of test)/mean IFU of organism control × 100. Results for the polymyxin B positive and penicillin G negative controls as well as the placebo control are also shown.
TABLE 2.
In vitro MCCs of gel formulations containing the peptide D2A21 against C. trachomatis
| D2A21 concn (%) | Time (min) | Methoda | MCCb | ECc (μM)
|
|
|---|---|---|---|---|---|
| 50% | 90% | ||||
| 2.0 | 120 | Mod. ultrafiltration | 0.2 mM (0.7 mg/ml) | 2.13 | 17.03 |
| 5 | Mod. ultrafiltration | No MCC achieved | 4.26 | 63.13 | |
| 0.5 | 90 | Standard | 0.2 mM (0.7 mg/ml) | 4.26 | 17.03 |
| 5 | Standard | No MCC achieved | 4.26 | 17.03 | |
| 0.1 | 120 | Standard | No MCC achieved | 1.7 | Neg |
| 0d | 120 | Mod. ultrafiltration | No MCC achieved | Neg | Neg |
| 5 | Mod. ultrafiltration | No MCC achieved | Neg | Neg | |
| 0d | 120 | Standard | No MCC achieved | Neg | Neg |
| 5 | Standard | No MCC achieved | Neg | Neg | |
Standard, standard MCC preinoculation method was used; Mod. ultrafiltration, modified ultrafiltration MCC method was used as described in Materials and Methods.
MCC when 100% killing was achieved.
EC, effective concentration that reduces infectivity by 50% or 90%, as indicated, compared to untreated control. Neg, no concentration tested achieved a 50 or 90% EC.
Placebo.
TABLE 3.
In vitro MCCs of peptides D2A21 and D4E1 against C. trachomatis
| Peptide | C. trachomatis serovar | MCCa | Minimal time (min) to MCC | ECb (μM)
|
|
|---|---|---|---|---|---|
| 50% | 90% | ||||
| D2A21 | D | 5 μM (18.32 μg/ml) | 120 | 2.5 | 2.5 |
| F | 5 μM (18.32 μg/ml) | 90 | 1.25 | 1.25 | |
| D4E1 | D | 7.5 μM (21.69 μg/ml) | 90 | 3.75 | 7.5 |
| F | 15 μM (43.38 μg/ml) | 120 | 3.75 | 7.5 | |
MCC when 100% killing was achieved.
EC, effective concentration that reduces infectivity by 50 or 90%, as indicated, compared to untreated control.
MCC in the presence of 10% human blood.
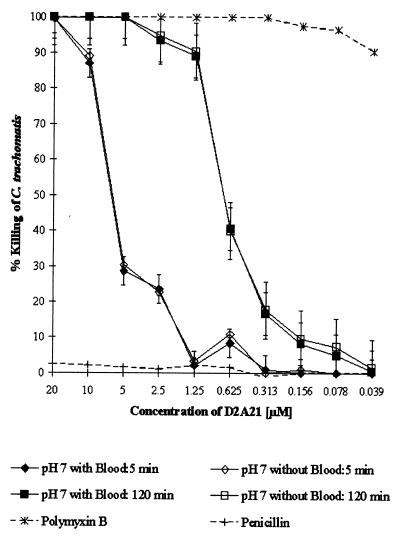
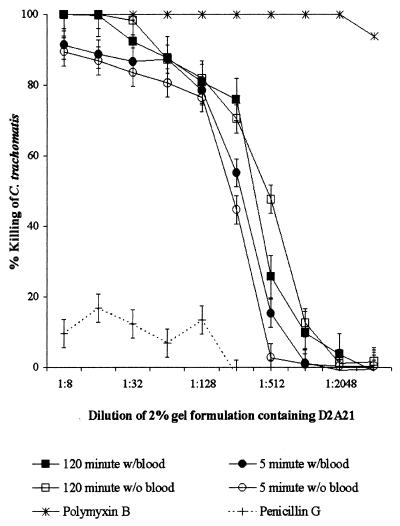
To assess the microbicidal activity of D2A21 in the presence of human blood, the peptide D2A21 and the four gel formulations containing D2A21 were tested in the preinoculation MCC assays in the presence and absence of 10% human blood. The MCCs of the peptide D2A21 and the 2 and 0.5% D2A21 gel formulations were not significantly affected by the presence of blood (Fig. 4 and 5). The 0.1% gel formulation did not achieve an MCC at any of the dilutions tested either in the presence or absence of blood. The placebo gel (containing 0% D2A21) had no activity in the presence or absence of blood.
FIG. 4.
Percent killing of C. trachomatis serovar D preexposed to the indicated concentrations of the peptide D2A21 for 5 and 120 min in the presence and absence of 10% whole human blood. Error bars indicate standard deviations of triplicate tests. Values for the organism and for polymyxin B and penicillin G controls were also determined in the MCC assay without the addition of 10% whole human blood.
FIG. 5.
Percent killing of C. trachomatis serovar D preexposed to dilutions of the 2% D2A21 gel formulation after 5 and 120 min in the presence and absence of 10% whole human blood. Error bars indicate standard deviations of triplicate tests. Values for the organism and for polymyxin B and penicillin G controls were also determined in the modified ultrafiltration MCC assay without the addition of 10% whole human blood.
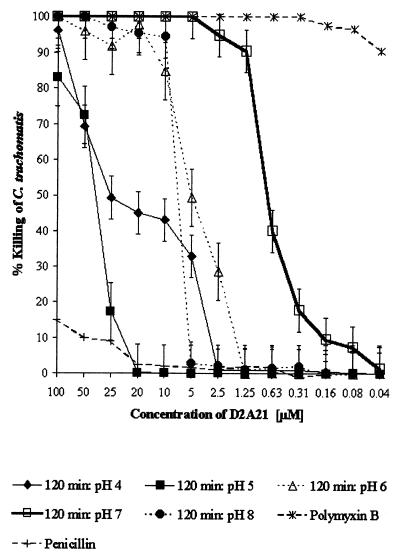
Alteration of pH and its effects on MCC.
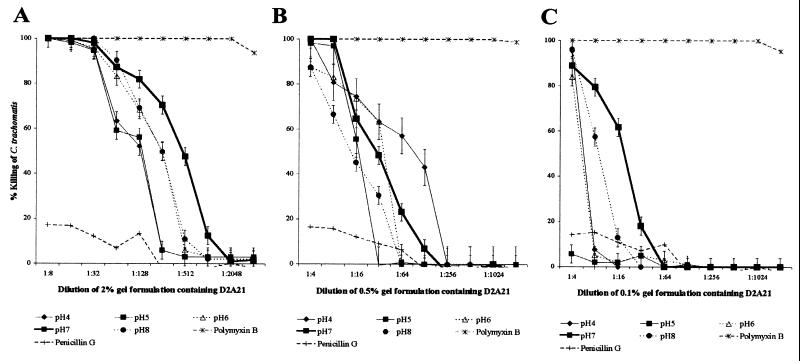
To assess the microbicidal activity of the peptide D2A21 and gel formulations containing D2A21 over a broad range of physiologically relevant pH values, the MCC activities were determined at pH 4, 5, 6, 7, and 8. The alteration of pH above and below pH 7 significantly reduced the MCC of the D2A21 peptide alone. The MCC of the D2A21 peptide at pH 7 after 120 min of exposure was 5 μM (18.3 μg/ml), and the MCC was reduced to 50 μM (183 μg/ml) at pH 8. At pH values below pH 7, the reduction in microbicidal activity was even greater. At pH 6, the MCC was reduced to 100 μM (0.4 mg/ml), and at pH 5 and pH 4 no MCC was achieved (Fig. 6). However, the gel formulation containing 2% D2A21 was not greatly affected by pH alterations. At pH 8, the MCC of the gel formulation containing 2% D2A21 increased by 1 dilution to 1:32 (0.2 mM [0.7 mg/ml]). Altering the pH of the 2% D2A21 gel formulation to either 4, 5, or 6 reduced the MCC by 1 twofold dilution to 1:8 (0.8 mM [2.7 mg/ml]) (Fig. 7A). The microbicidal activities of the 0.5% gel formulation containing D2A21 were still significantly reduced at pHs above and below pH 7 (Fig. 7B). Altering the pH of the 0.5% D2A21-containing gel to pH 4 reduced the MCC by 1 twofold dilution to 1:4 (0.4 mM [1.4 mg/ml]) (Fig. 7B). The MCC of the 0.5% D2A21 gel formulation at pH 7 remained the same, with complete killing achieved at a dilution of 1:8 (0.2 mM [0.7 mg/ml]). At pH 5, 6, and 8, however, the gel containing 0.5% D2A21 did not achieve an MCC. The 0.1% gel formulation containing D2A21 did not achieve an MCC at any of the pH values (Fig. 7C).
FIG. 6.
D2A21 activity at pH 4, 5, 6, 7, and 8 at 120 min. The percent killing of C. trachomatis serovar D by the peptide D2A21 is shown at each pH value, with standard deviations (from triplicate tests) indicated by error bars for each concentration. Values were calculated in comparison to that of the organism control at the same pH value. The standard MCC assay protocol was followed.
FIG. 7.
Antichlamydial activities of gel formulations containing 2% D2A21 (A), 0.5% D2A21 (B), or 0.1% D2A21 (C) at pH 4, 5, 6, 7, and 8 at 120 min. The percent killing of C. trachomatis serovar D by each of the gel formulations containing D2A21 is shown at each pH value, with standard deviations (from triplicate tests) indicated by error bars for each concentration. Values were calculated in comparison to that of the organism control at the same pH value. Results for the polymyxin B positive and penicillin G negative controls are also shown. The modified ultrafiltration MCC method was used with the 2% gel, and the standard MCC protocol was followed for the 0.5 and 0.1% gels.
Postinoculation MIC assay.
To assess the putative therapeutic effect of D2A21 and whether the peptide can enter eukaryotic cells, cells previously infected with C. trachomatis were overlaid with dilutions of D2A21 in EMEM-C and incubated for 48 h. D2A21 did not inhibit the formation of chlamydial inclusions at any of the concentrations tested (data not shown).
E. coli MCC assay.
For comparison purposes, the in vitro peptide MCC was determined against an E. coli reference strain. The peptide D2A21 achieved an MCC of 12.5 μM (46 μg/ml) against the reference E. coli strain 25922 (Table 4) after 2 h of exposure. Shorter times were not tested.
TABLE 4.
Microbicidal activity of D2A21 against E. coli after 120 min of exposure
| Concn of peptidea
|
Mean CFU (SD)b | % Reduction in CFU | |
|---|---|---|---|
| μM | μg/ml | ||
| 100 | 366 | 0 (0) | 100 |
| 50 | 183 | 0 (0) | 100 |
| 25 | 92 | 0 (0) | 100 |
| 12.5 | 46 | 8 × 105 (3 × 105) | 97.5 |
| 6.25 | 23 | 1.1 × 105 (1.8 × 105) | 96.7 |
| 3.125 | 11.4 | 6.2 × 106 (1.8 × 106) | 81.1 |
| 1.565 | 5.7 | 9 × 106 (1.4 × 106) | 73.2 |
| 0 | 0 | 3.3 × 107 (1.2 × 106) | 0 |
Values represent the concentrations of peptide directly exposed to E. coli strain 25922.
Assays were performed in triplicate, and results were averaged.
Examination of C. trachomatis EBs exposed to the D2A21 peptide by electron microscopy.
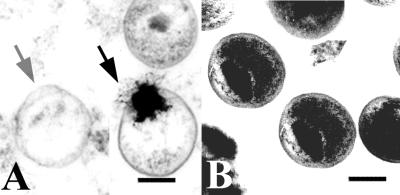
C. trachomatis EBs exposed to 5.0 μM (18 μg/ml) D2A21 for 90 min were examined by transmission electron microscopy. After exposure to the peptide, chlamydial EBs appeared as ghosts with only an outer membrane and no cytoplasmic contents (Fig. 8A). Alternatively, organisms appeared to be leaking their electron-dense cytoplasmic contents (Fig. 8A). It appears that the inner membrane is disrupted, but the outer membrane remains relatively intact.
FIG. 8.
Transmission electron micrographs of C. trachomatis serovar D exposed to peptide D2A21 for 90 min. (A) Organisms treated with D2A21 compared to the untreated control appear as if the inner membrane had lost its structural integrity (gray arrow) or they are in the process of leaking their cytoplasmic contents (black arrow). (B) Untreated organisms incubated in SPG only and processed for microscopy. It is important to note the intact outer membrane structures and electron-dense cytoplasmic mass. The figures shown are representative of the entire field. Electron micrographs were scanned on an Agfa Arcus II flatbed scanner in Adobe Photoshop, version 5.0. Bar = 0.5 μm.
DISCUSSION
A number of potential microbicides, such as the detergents chlorhexidine gluconate, nonoxynol-9, benzalkonium chloride, and sodium dodecyl sulfate, synthetic lipids, fatty acids, and protegrin and defensin peptides, have been examined for their potential topical activities against STI-causing pathogens (2, 11, 16–18, 36). However, some of these compounds such as the detergent nonoxynol-9 have significant cytotoxicity, and others such as some defensins, protegrins, and clavanins are inactivated in the presence of blood or have reduced antimicrobial activities at various pHs (19, 24, 28, 36). Thus, there is a need to evaluate other microbicides.
We have shown that the peptides D2A21 and D4E1 and the gel formulations containing 2 and 0.5% D2A21 effectively kill C. trachomatis EBs at concentrations and under conditions that could be achieved in the vaginal vault and anogenital tract. However, it is important to separate the effects of the compounds on the host cells from their activities on C. trachomatis, since chlamydiae require healthy host cells in which to replicate. Our results indicate that the D2A21 and D4E1 peptides and the gels containing D2A21 are active because they kill C. trachomatis and not because they are toxic to the tissue culture cells. To measure the cytotoxic effect of the peptides and gel formulations on uninfected McCoy cell monolayers, the AlamarBlue cytotoxicity assay was used. This assay is more sensitive than visual determination and is equivalent to other cytotoxicity assays such as neutral red uptake, MTT (3-[4-5-dimethylthiazol-2-yl]-2-5-diphenyl bromide tetrazolium bromide), or chromium-51 release (8, 23).
The AlamarBlue cytotoxicity assay showed that of all the peptides and gel formulations tested in these studies, only the 2% D2A21 gel had significant cytotoxicity in the chlamydial MCC assay. By incorporating an ultrafiltration step, we eliminated the McCoy cell cytotoxicity in our in vitro assay and showed that the MCC of 2% D2A21 gel was 0.2 mM (0.7 mg/ml). A gel formulation of 1% D2A21 is currently being evaluated for cytotoxicity and chlamydicidal activity in order to find the peptide gel formulation that results in the least cytotoxicity and maximum antimicrobial activity. The lack of inhibition of C. trachomatis in a postinoculation MIC assay suggests that D2A21 does not penetrate eukaryotic cells, but this needs to be confirmed by more specific methods. Cytotoxicity studies in animals and eventually in humans will be needed to confirm that the final D2A21 gel formulation possesses no cytotoxicity.
There are a variety of environmental conditions in the vagina and rectum that a topical microbicide might encounter. These include the presence of blood and a wide range of pH values. Blood may be present in the rectum as a result of mucosal infection or traumatic receptive anal intercourse and in the vaginal vault during menstruation (3, 6, 14, 34, 37). It has been shown that some peptides, including some defensins, are inactivated in the presence of serum (35). The normal pH in the vagina is pH 4, but after the deposition of semen, it may reach pH 8. The normal rectal pH range in adult males is between pH 6 and 7 but has been shown to be as high as pH 8 (14, 21, 25, 31). Ideally, a topical microbicide must thus retain its activity over a broad range of pH values. Our results suggest that the peptide D2A21, although not significantly affected by the presence of blood, shows decreased or no activity at pH values above and below pH 7. We have also shown that this reduction of microbicidal activity at various pH values can be eliminated or significantly reduced when the peptide is formulated in a gel. The 2% gel formulation effectively eliminated this reduced activity. The 0.5% gel formulations containing D2A21 also reduced this effect but still failed to achieve complete killing after 120 min of exposure at pH 8. Therefore, the 2% gel formulation containing D2A21 may be more effective when used as a topical microbicide for the prevention of C. trachomatis infection. However, the 2% gel formulation should be examined for toxicity on vaginal, cervical, and rectal tissues both in an animal model and in humans. As mentioned above, a 1% D2A21 gel formulation is also being evaluated for chlamydicidal activity and toxicity.
The peptides alone had very good activities, with the MCC of D2A21 being 5 μM (18.3 μg/ml) and that of D4E1 being 7.5 μM (21.7 μg/ml), making them ideal candidates for an effective microbicide. The activity of the D2A21 peptide in gel formulations was higher (MCC of the 2% gel, 0.2 mM [0.7 mg/ml]); MCC of the 0.5% gel, 0.2 mM [0.7 mg/ml]). These results demonstrate that it is very important to test both peptides alone and in gel formulations since these gel excipients had an inhibitory effect on the peptides’ activities. D2A21 is currently being reformulated in a gel that will allow full expression of its inherent activity.
The mode of action of these peptides is not completely understood. Past studies on other cationic peptides have shown that some cationic peptides bind lipopolysaccharide and permeabilize the outer membrane of gram-negative bacteria, allowing entry via self-promoted uptake (4, 10, 12). Other studies show that similar cationic peptides form porins or channels in planar lipid bilayer membrane models, possibly allowing a route for the passage of ions or the peptide through the bacterial membrane (4, 12). Our electron microscopic examination shows that exposure of C. trachomatis EBs to the peptide D2A21 results in membrane lysis or disruption, but further studies to evaluate the mechanisms involved are in progress.
In conclusion, these in vitro results indicate that the antimicrobial peptides D2A21 and D4E1 are effective microbicides at concentrations achievable in vivo. Both the D2A21 and D4E1 peptides have in vitro activities against human immunodeficiency virus, N. gonorrhoeae, Gardnerella vaginalis, Trichomonas vaginalis, and Candida albicans and are spermicidal (J. M. Jaynes, personal communication, 1996) at concentrations at or below the MCC for C. trachomatis. The formulated D2A21 product is microbicidal at achievable concentrations, and its activity is maintained at various pHs and in the presence of human blood. The 2% gel demonstrated significant cytotoxicity to McCoy cells in our in vitro C. trachomatis assays, but specific evaluations of the efficacy and cytotoxicity of formulated 1 and 2% D2A21 products in humans are in progress. Further assessment of the topical activities of these peptides against other STI pathogens, as well as against normal flora, are also in progress.
Acknowledgments
This work was supported by Public Health Service grant #PO1 AI 37830 from the National Institutes of Health and Demegen, Inc.
We thank Peter Cummings for the transmission electron microscopy work.
REFERENCES
- 1.Bechinger, B. 1997. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197–211. [DOI] [PubMed] [Google Scholar]
- 2.Bergsson, G., J. Arnfinnsson, S. M. Karlsson, O. Steingrimsson, and H. Thormar. 1998. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42:2290–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisvert, J. F., L. A. Koutsky, R. J. Suchland, and W. E. Stamm. 1999. Clinical features of Chlamydia trachomatis rectal infection by serovar among homosexually active men. Sex. Transm. Dis. 26:392–398. [DOI] [PubMed] [Google Scholar]
- 4.Boman, H. G. 1998. Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand J. Immunol 48:15–25. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Summary of notifiable diseases, United States, 1998. Morb. Mortal. Wkly. Rep. 47:11–23. [PubMed] [Google Scholar]
- 6.El-Attar, S. M., and D. V. Evans. 1999. Anal warts, sexually transmitted diseases, and anorectal conditions associated with human immunodeficiency virus. Prim. Care 26:81–100. [DOI] [PubMed] [Google Scholar]
- 7.Elias, C. J., and L. L. Heise. 1994. Challenges for the development of female-controlled vaginal microbicides. AIDS 8:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Fields, R. D., and M. V. Lancaster. 1993. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 11:48–50. [PubMed] [Google Scholar]
- 9.Gagne, N., H. Cormier, R. F. Omar, A. Desormeaux, P. Gourde, M. J. Tremblay, J. Juhasz, D. Beauchamp, J. E. Rioux, and M. G. Bergeron. 1999. Protective effect of a thermoreversible gel against the toxicity of nonoxynol-9. Sex. Transm. Dis. 26:177–183. [DOI] [PubMed] [Google Scholar]
- 10.Giacometti, A., O. Cirioni, G. Greganti, M. Quarta, and G. Scalise. 1998. In vitro activities of membrane-active peptides against gram-positive and gram-negative aerobic bacteria. Antimicrob. Agents Chemother. 42:3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang, P. M., and H. J. Vogel. 1998. Structure-function relationships of antimicrobial peptides. Biochem. Cell. Biol. 76:235–246. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. 1996. The hidden epidemic: confronting sexually transmitted diseases. National Academy Press, Washington, D.C.
- 14.Jones, D. J., and B. P. Goorney. 1992. ABC of colorectal diseases. Sexually transmitted diseases and anal papillomas. Br. Med. J. 305:820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, M. Alary, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95–102. [DOI] [PubMed] [Google Scholar]
- 16.Lampe, M. F., L. M. Ballweber, C. E. Isaacs, S. J. Klebanoff, and W. E. Stamm. 1998. Susceptibility of Chlamydia trachomatis to novel topical microbicides. In R. Stephens, G. I. Byrne, G. Christiansen, I. N. Clarke, J. T. Grayston, R. G. Rank, G. L. Ridgeway, P. Saikku, J. Schachter, and W. E. Stamm (ed.), Chlamydial infections: proceedings of the Ninth International Symposium on Human Chlamydial Infection, Napa, Calif. International Chlamydia Symposium, San Francisco, Calif.
- 17.Lampe, M. F., L. M. Ballweber, C. E. Isaacs, D. L. Patton, and W. E. Stamm. 1998. Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk. Antimicrob. Agents Chemother. 42:1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampe, M. F., L. M. Ballweber, and W. E. Stamm. 1998. Susceptibility of Chlamydia trachomatis to chlorhexidine gluconate gel. Antimicrob. Agents Chemother. 42:1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, I. H., Y. Cho, and R. I. Lehrer. 1997. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 65:2898–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons, J. M., and J. I. Ito, Jr. 1995. Reducing the risk of Chlamydia trachomatis genital tract infection by evaluating the prophylactic potential of vaginally applied chemicals. Clin. Infect. Dis. 21(Suppl. 2):S174–S177. [DOI] [PubMed] [Google Scholar]
- 21.McNeil, N. I., K. L. Ling, and J. Wager. 1987. Mucosal surface pH of the large intestine of the rat and of normal and inflamed large intestine in man. Gut 28:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, A. J., W. D. Beazley, M. C. Bibby, and D. A. Devine. 1996. Antimicrobial activity of cecropins. J. Antimicrob. Chemother. 37:1077–1089. [DOI] [PubMed] [Google Scholar]
- 23.Page, B., M. Page, and C. Noel. 1993. A new fluorometric assay for cytotoxicity measurements in vitro. Int. J. Oncol. 3:473–476. [PubMed] [Google Scholar]
- 24.Patton, D. L., G. G. Kidder, Y. C. Sweeney, L. K. Rabe, and S. L. Hillier. 1999. Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina). Am. J. Obstet. Gynecol. 180:1080–1087. [DOI] [PubMed] [Google Scholar]
- 25.Pye, G., D. F. Evans, S. Ledingham, and J. D. Hardcastle. 1990. Gastrointestinal intraluminal pH in normal subjects and those with colorectal adenoma or carcinoma. Gut 31:1355–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saberwal, G., and R. Nagaraj. 1994. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim. Biophys. Acta 1197:109–131. (Erratum, 1235:159, 1995.) [DOI] [PubMed] [Google Scholar]
- 27.Sokal, D. C., and P. L. Hermonat. 1995. Priorities for vaginal microbicide research. Am. J. Public Health 85:737–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327–331. [DOI] [PubMed] [Google Scholar]
- 29.Stamm, W. E., and R. Suchland. 1986. Antimicrobial activity of U-70138F (paldimycin), roxithromycin (RU 965), and ofloxacin (ORF 18489) against Chlamydia trachomatis in cell culture. Antimicrob. Agents Chemother. 30:806–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephens, R. (ed.). 1999. Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 31.Thompson, C. I., A. J. MacAulay, and I. W. Smith. 1989. Chlamydia trachomatis infections in the female rectums. Genitourin. Med. 65:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Damme, L., S. Niruthisard, R. Atisook, K. Boer, L. Dally, M. Laga, J. M. Lange, M. Karam, and J. H. Perriens. 1998. Safety evaluation of nonoxynol-9 gel in women at low risk of HIV infection. AIDS 12:433–437. [DOI] [PubMed] [Google Scholar]
- 33.Wachinger, M., A. Kleinschmidt, D. Winder, N. von Pechmann, A. Ludvigsen, M. Neumann, R. Holle, B. Salmons, V. Erfle, and R. Brack-Werner. 1998. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 79:731–740. [DOI] [PubMed] [Google Scholar]
- 34.Wexner, S. D. 1990. Sexually transmitted diseases of the colon, rectum, and anus. The challenge of the nineties. Dis. Colon Rectum 33:1048–1062. [DOI] [PubMed] [Google Scholar]
- 35.Workowski, K. A., C. E. Stevens, R. J. Suchland, K. K. Holmes, D. A. Eschenbach, M. B. Pettinger, and W. E. Stamm. 1994. Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin. Infect. Dis. 19:756–760. [DOI] [PubMed] [Google Scholar]
- 36.Yasin, B., S. S. Harwig, R. I. Lehrer, and E. A. Wagar. 1996. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 64:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zollner, B., H. H. Feucht, H. Koch, L. Iske, G. Oehler, H. J. Stellbrink, and R. Laufs. 1993. Isolation of Chlamydia trachomatis from the lower digestive tract. Infection 21:318–320. [DOI] [PubMed] [Google Scholar]