Abstract
Previous studies have demonstrated that fluoroquinolone area under the curve (AUC)/MIC ratios of 30 to 50 are sufficient to eradicate pneumococci from in vitro pharmacokinetic models (IVPMs). However, more systematic studies of the impact of AUC/MIC ratios on the antipneumococcal activities of fluoroquinolones are needed. In the present study, a two-compartment IVPM was used to evaluate the impact of AUC/MIC ratios on the pharmacodynamics of gatifloxacin against four strains of Streptococcus pneumoniae. Gatifloxacin MICs were 0.4 to 1 μg/ml, whereas levofloxacin MICs were 1.8 to 3.2 μg/ml. Since both peak concentration/MIC (peak/MIC) and AUC/MIC ratios affect fluoroquinolone pharmacodynamics, logarithmic-phase cultures (5 × 107 CFU/ml) were exposed to gatifloxacin at constant peak/MIC ratios of 2:1 to 3:1 at 0 and 24 h, elimination half-lives were varied to provide a range of AUC/MIC ratios, and changes in viable counts were measured over 30 h. As a comparison, levofloxacin was evaluated at similar peak/MIC ratios and at AUC/MIC ratios of 30 to 38. For each strain, killing rates through 4 to 8 h were similar since peak/MIC ratios were kept constant. However, continued killing and eradication were observed only when gatifloxacin AUC/MIC ratios were 27 to 48. Levofloxacin also provided eradication. In contrast, substantial regrowth was observed in most experiments when gatifloxacin AUC/MIC ratios were 9 to 24. These data provide further support that fluoroquinolone AUC/MIC ratios of approximately 30 or higher can be sufficient for eradication of pneumococci from IVPMs. Furthermore, the overall impact of the AUC/MIC ratio was not influenced by the strain evaluated or its susceptibility to gatifloxacin. Further studies with other fluoroquinolones and pneumococci that exhibit wider ranges of susceptibilities are warranted. In addition, similar studies with higher peak/MIC ratios are needed to better define the impact of AUC/MIC ratios and peak/MIC ratios on the antipneumococcal pharmacodynamics of fluoroquinolones.
Clinical data and data from in vitro pharmacodynamic studies have suggested that the ratio of the area under the concentration curve (AUC) divided by the MIC (the AUC/MIC ratio) is an important pharmacodynamic parameter that influences the outcome of fluoroquinolone therapy. Although initial clinical observations with ciprofloxacin in the treatment of serious infections caused by gram-negative bacteria suggested that AUC/MIC ratios of 125 or greater were needed to optimize the clinical response (8), data from studies with in vitro pharmacokinetic models (IVPMs) have suggested that AUC/MIC ratios in the range of 30 to 50 are sufficient to achieve eradication of Streptococcus pneumoniae with various fluoroquinolones (9–11). On the basis of these published observations, it has been suggested that the minimum AUC/MIC ratio required to achieve eradication from IVPMs and to potentially provide clinical efficacy with fluoroquinolones is approximately 30. However, these initial studies were narrow in their focus and were not designed to systematically evaluate the impacts of AUC/MIC ratios on the pharmacodynamics of fluoroquinolones. In order to truly understand the relationship between the AUC/MIC ratios of fluoroquinolones and pharmacodynamic interactions with S. pneumoniae, a systematic evaluation of the pharmacodynamics of fluoroquinolones against pneumococci over a range of AUC/MIC ratios is required.
In the present study, a two-compartment IVPM was used to expose clinical isolates of S. pneumoniae to gatifloxacin over a range of AUC/MIC ratios from approximately 10 to 50 and to evaluate the impacts of the AUC/MIC ratios on the eradication of pneumococci from the model. As a control, the pharmacodynamics of levofloxacin at AUC/MIC ratios of approximately 30 were also studied. Since fluoroquinolones are concentration-dependent bactericidal agents and, thus, peak concentration/MIC (peak/MIC) ratios influence their pharmacodynamics, peak/MIC ratios were kept constant in these studies and AUC/MIC ratios were varied by altering the elimination half-lives.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Four isolates of S. pneumoniae were selected for use in the present study. Two strains were penicillin susceptible and two strains were penicillin intermediate. The susceptibilities of these isolates to gatifloxacin and levofloxacin are shown in Table 1. Logarithmic-phase cultures were prepared by suspending 10 colonies from a 14-h culture on Trypticase soy agar supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) into 6 ml of Todd-Hewitt broth (THB; Unipath/Oxoid, Ogdensburg, N.Y.). After 10 h of incubation at 37°C in 5% CO2, viable bacterial counts ranged from 1 × 108 to 5 × 108 CFU/ml.
TABLE 1.
Susceptibility of S. pneumoniae to gatifloxacin and levofloxacin
| S. pneumoniae strain | Penicillin susceptibility | MIC (μg/ml)a
|
|
|---|---|---|---|
| Gatifloxacin | Levofloxacin | ||
| 256 | Susceptible | 1.0 | 3.2 |
| 257 | Susceptible | 0.6 | 2.6 |
| 313 | Intermediate | 0.4 | 2.0 |
| 314 | Intermediate | 0.4 | 1.8 |
MICs were measured by the broth macrodilution method in THB by using 0.2-μg/ml increments.
Antibiotic preparations.
Gatifloxacin powder was supplied by Bristol-Myers Squibb. Levofloxacin powder was supplied by R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J. Gatifloxacin and levofloxacin powders were dissolved in 0.2 ml of 0.1 M NaOH, diluted to the final volume with distilled water, and sterilized by passage through a 0.20-μm-pore-size Acrodisc syringe filter membrane (Gelman Sciences, Ann Arbor, Mich.).
Antimicrobial susceptibility testing.
Susceptibility tests with gatifloxacin and levofloxacin were performed by the broth macrodilution methodology by the procedure recommended by the National Committee for Clinical Laboratory Standards (13), with the following exceptions. THB was used as the test medium since it was the medium used for the pharmacodynamic studies. In addition, to more accurately define the AUC/MIC ratios, MICs were evaluated by using 0.2-μg/ml increments rather than the standard twofold dilution methodology.
IVPM.
The IVPM used in the present study was a modification (12) of the original model described by Blaser and colleagues (1). The hollow-fiber cartridges (model BR130; Unisyn Fibertech, San Diego, Calif.) used in the studies consisted of 2,250 hollow cellulose acetate fibers contained within a polycarbonate housing, with each fiber having within its wall pores that allow passage of a molecule with a molecular weight of 30,000. The surface area of exchange between the hollow fibers and the extracapillary space (peripheral compartment) was 1.5 ft2. Medium containing antibiotic was pumped through the lumens of the fibers at a flow rate of 20 ml/min with Masterflex computerized peristaltic pumps (model 7550-90; Cole-Parmer Instrument Company, Vernon Hills, Ill.) and Easy-Load pump heads (model 7518-00; Cole-Parmer Instrument Company). In addition, the bacterial culture within the peripheral compartment was continuously circulated with similar peristaltic pumps at a rate of 20 ml/min through a loop of silicone tubing attached to two ports that entered and exited the peripheral compartment. The initial volume of culture that circulated through the peripheral compartment and the loop of silicone tubing was 35 to 40 ml. When samples from the peripheral compartment were required, 0.5-ml volumes were removed through a four-way sterile stopcock (Medex, Hilliard, Ohio) positioned within the loop of silicone tubing. The volume of THB within the central reservoir varied depending upon the elimination half-life, such that the rates of dilution and elimination could be set at the minimum allowed by the pumps (0.7 ml/min). In drug-free control experiments, the volume of THB in the central reservoir was 500 ml and the flow rates for the addition of fresh media and elimination from the central reservoir were 2 ml/min.
Pharmacokinetic profiles.
Peak concentrations of gatifloxacin of 1.2 to 2.5 μg/ml were targeted in these studies to provide peak/MIC ratios of approximately 2.5:1 for S. pneumoniae 256, 2:1 for S. pneumoniae 257, and 3:1 for S. pneumoniae 313 and 314. Elimination half-lives of 4, 6, 12, and 24 h were simulated to provide AUC/MIC ratios of approximately 14, 21, 33, and 44, respectively, for S. pneumoniae 256. Elimination half-lives of 5, 8, 12, and 24 h were simulated to provide AUC/MIC ratios of approximately 14, 21, 27, and 36, respectively, for S. pneumoniae 257. Elimination half-lives of 3, 5, 8, and 12 h were simulated to provide AUC/MIC ratios of approximately 13, 21, 31, and 40, respectively, for S. pneumoniae 313 and 314.
Peak concentrations of levofloxacin of approximately 3.5 to 6.5 μg/ml were targeted to provide peak/MIC ratios of 2:1 for the S. pneumoniae strains tested in the present study. An elimination half-life of 2 h was simulated in each experiment with levofloxacin to provide AUC/MIC ratios of approximately 30.
The actual AUC/MIC ratios to which the S. pneumoniae strains were exposed varied depending upon the peak concentration actually achieved in each experiment. The concentrations of gatifloxacin and levofloxacin in the IVPM were measured by a disk diffusion bioassay with a susceptible strain of Escherichia coli (6). The lower limit of sensitivity of this bioassay was 0.4 μg/ml, with a linear range up to 1.2 μg/ml. Samples were diluted before testing to provide two points within the range of the linear standard curve of the bioassay. The AUCs over 24 h (AUC0–24s) were calculated by the trapezoidal rule. The AUC/MIC ratios were calculated by dividing the AUC0–24 by the MICs for specific strains of S. pneumoniae (16).
Pharmacodynamic studies in IVPM.
Pharmacodynamic studies with S. pneumoniae were evaluated in THB at 37°C in ambient air. Logarithmic-phase cultures (5 × 107 CFU/ml) were introduced into the peripheral compartment of the IVPM and were exposed to the peak concentrations of gatifloxacin or levofloxacin that were approximately twofold to threefold above the MIC. Gatifloxacin and levofloxacin were also dosed at the same concentrations at 24 h. Elimination half-lives were varied in the experiments with gatifloxacin to provide a range of AUC/MIC ratios from approximately 10 to 50. Since gatifloxacin and other fluoroquinolones kill bacteria in a concentration-dependent manner, AUC/MIC ratios were varied by altering rates of elimination rather than peak concentrations. Therefore, any differences in the pharmacodynamics of killing could be related to differences in AUC/MIC ratios and the times that the concentrations remained above the MIC, not the peak/MIC ratios. As a comparative positive control in these studies, AUC/MIC ratios of approximately 30 were simulated with levofloxacin.
To evaluate pharmacodynamic interactions, samples were removed from the peripheral compartment of the IVPM at 0, 2, 4, 8, 12, 24, and 30 h and viable bacterial counts were measured by plating serial 10-fold dilutions of each sample into Todd-Hewitt agar (BBL) and incubating the plates overnight at 37°C in 5% CO2. Antibiotic carryover was prevented by first incubating samples taken from the IVPM with antibiotic-removal beads for 15 min (18). The lowest dilution plated was 0.1 ml of undiluted sample from the peripheral compartment. Therefore, the lowest level of detection was 1 colony per plate or 10 CFU/ml. To detect the presence of mutants that exhibited decreased susceptibility to fluoroquinolones, at 30 h samples were also plated into Todd-Hewitt agar containing gatifloxacin or levofloxacin at a concentration fourfold the MIC.
RESULTS
Characterization of test strains.
The MICs of gatifloxacin and levofloxacin for the four S. pneumoniae isolates are shown in Table 1. The MICs of gatifloxacin ranged from 0.4 to 1 μg/ml, and the MICs of levofloxacin ranged from 1.8 to 3.2 μg/ml.
Pharmacodynamic studies.
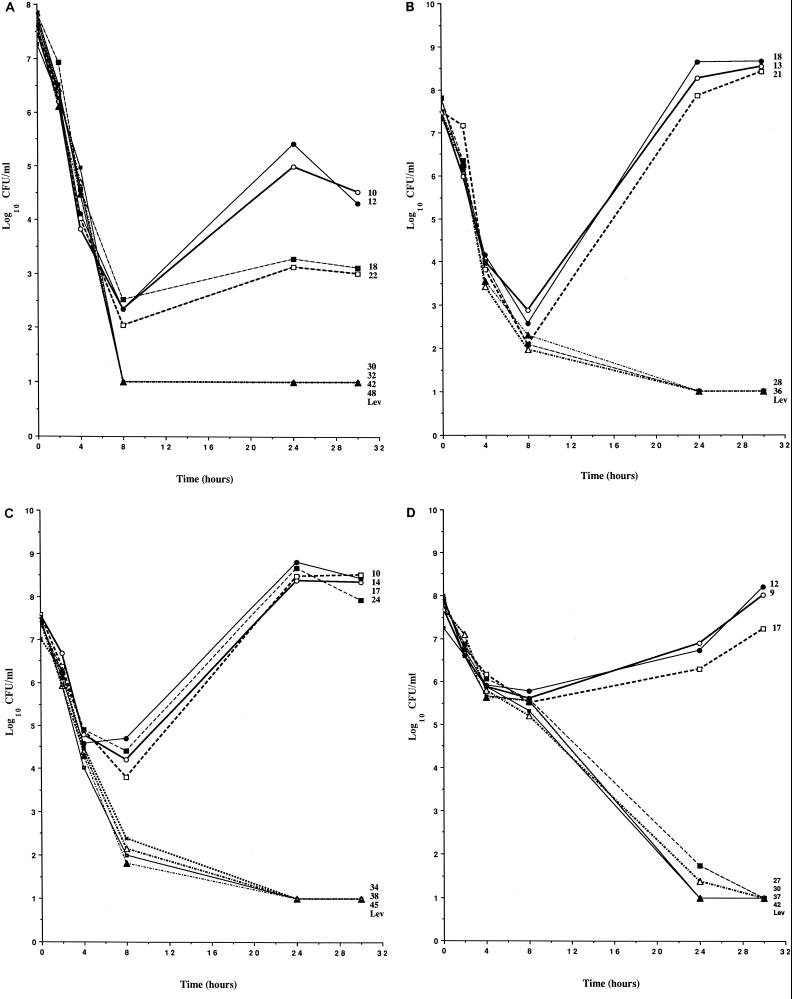
The pharmacodynamics of gatifloxacin and levofloxacin against the S. pneumoniae strains are shown in Fig. 1. The actual AUC/MIC ratios to which the S. pneumoniae strains were exposed in each experiment are shown to the right of the datum lines. Drug-free control cultures grew to a maximum of 1 × 108 to 5 × 108 CFU/ml by 8 to 12 h, and viable counts remained steady throughout the remainder of the 30-h experimental period (data not shown).
FIG. 1.
Pharmacodynamics of gatifloxacin and levofloxacin against S. pneumoniae 256 (A), S. pneumoniae 257 (B), S. pneumoniae 313 (C), and S. pneumoniae 314 (D). Each datum point represents the number of viable bacteria (log10 CFU per milliliter) for single experiments, and numbers to the right of the datum lines represent the actual AUC/MIC ratios to which the S. pneumoniae strains were exposed in each experiment. LEV, pharmacodynamics of the levofloxacin comparative control in each experiment.
S. pneumoniae 256.
Cultures of S. pneumoniae 256 were exposed to gatifloxacin at peak/MIC ratios of approximately 2.5:1 in all experiments, which provided similar rates of bacterial killing over the initial 6 h (Fig. 1A). By 6 h, the viable counts had fallen approximately 4 log units in all experiments. Between 8 and 30 h, however, the effects of varying the AUC/MIC ratios became apparent. When the AUC/MIC ratios were ≥30, total eradication (i.e., the point at which the viable counts fell below the limit of detection of 10 CFU/ml) of the S. pneumoniae strain was observed. In contrast, when AUC/MIC ratios were ≤22, viable counts either changed very little between 8 and 24 h or increased 2 to 3 log units by 24 h. In relation to the time that the concentration was above the MIC (T>MIC), eradication of S. pneumoniae 256 required that concentrations of gatifloxacin remain above the MIC for more than 8 h, as eradication was not achieved when concentrations were above the MIC for ≤8 h. No mutants with decreased susceptibility to gatifloxacin were detected in these experiments. In studies with levofloxacin at a peak/MIC ratio of 2:1 and an AUC/MIC ratio of 33, the initial rate of bacterial killing was similar to that obtained with gatifloxacin and the bacterial inoculum was eradicated (Fig. 1A).
S. pneumoniae 257.
Cultures of S. pneumoniae 257 were exposed to gatifloxacin at peak/MIC ratios of approximately 2:1 in all experiments, which provided similar rates of bacterial killing over the initial 8 h (Fig. 1B). By 8 h, the viable counts in all experiments had fallen approximately 5 log units. When the AUC/MIC ratios were 28 and 36, the viable counts continued to decline after 8 h until the inocula were eradicated below the limit of detection of 10 CFU/ml. In contrast, when AUC/MIC ratios were ≤21, viable counts increased rapidly between 8 and 24 h until they exceeded the counts in the original inocula. Very little change in viable counts was observed between 24 and 30 h. In relation to T>MIC, eradication of S. pneumoniae 257 required that concentrations of gatifloxacin remain above the MIC for more than 8 h, as eradication was not achieved when concentrations were above the MIC for ≤8 h. No mutants with decreased susceptibility to gatifloxacin were detected in these experiments. In studies with levofloxacin at a peak/MIC ratio of 2:1 and an AUC/MIC ratio of 30, the initial rate of bacterial killing was similar to that obtained with gatifloxacin and the bacterial inoculum was eradicated (Fig. 1B).
S. pneumoniae 313.
Cultures of S. pneumoniae 313 were exposed to gatifloxacin at peak/MIC ratios of approximately 3:1 in all experiments, which provided similar rates of bacterial killing over the initial 4 h (Fig. 1C). By 4 h, the viable counts in all experiments had fallen approximately 3 log units. When the AUC/MIC ratios were ≥34, the viable counts continued to rapidly decline after 4 h until the inocula were eradicated (were below the limit of detection of 10 CFU/ml) by 24 h. In contrast, when AUC/MIC ratios were ≤24, decreases in viable counts slowed between 4 and 8 h, and then the viable counts rapidly increased between 8 and 24 h until they exceeded the counts in the original inocula. Very little change in viable counts was observed between 24 and 30 h. In relation to T>MIC, eradication of S. pneumoniae 313 required that concentrations of gatifloxacin remain above the MIC for more than 8 h, as eradication was not achieved when concentrations were above the MIC for ≤8 h. No mutants with decreased susceptibility to gatifloxacin were detected in these experiments. In studies with levofloxacin at a peak/MIC ratio of 2:1 and an AUC/MIC ratio of 38, the initial rate of bacterial killing was similar to that obtained with gatifloxacin and the bacterial inoculum was eradicated (Fig. 1C).
S. pneumoniae 314.
Cultures of S. pneumoniae 314 were exposed to gatifloxacin at peak/MIC ratios of approximately 3:1 in all experiments, which provided similar rates of bacterial killing over the initial 8 h (Fig. 1D). By 8 h, the viable counts in all experiments had fallen approximately 2 log units. When the AUC/MIC ratios were ≥27, the viable counts continued to decline after 4 h until the inocula were eradicated (were below the limit of detection of 10 CFU/ml) by 24 or 30 h. In contrast, when the AUC/MIC ratios were ≤17, bacteriostasis was observed between 4 and 8 h, and then the viable counts increased slightly between 8 and 24 h. By 30 h, the viable counts were equal to or above the original inocula. In relation to the T>MIC, eradication of S. pneumoniae 314 required that concentrations of gatifloxacin remain above the MIC for more than 8 h, as eradication was not achieved when concentrations were above the MIC for ≤8 h. No mutants with decreased susceptibility to gatifloxacin were detected in these experiments. In studies with levofloxacin at a peak/MIC ratio of 2:1 and an AUC/MIC ratio of 32, the initial rate of bacterial killing was similar to that obtained with gatifloxacin and the bacterial inoculum was eradicated (Fig. 1D).
DISCUSSION
Fluoroquinolones are concentration-dependent bactericidal agents that exhibit dose-dependent killing activity (5, 17). Therefore, the peak/MIC ratio plays an important role in their pharmacodynamic interactions with bacteria. In addition to the influence of the peak/MIC ratio, the AUC/MIC ratio also affects the pharmacodynamics and clinical efficacies of fluoroquinolones (8, 15). Which of these two parameters is most statistically associated with a positive clinical outcome depends upon the study and the patient populations. Although observations from clinical studies with ciprofloxacin for the treatment of serious infections caused by gram-negative bacteria have suggested that the AUC/MIC ratio is the most important pharmacodynamic parameter that affects the clinical outcome (8), data from clinical studies with levofloxacin for the treatment of respiratory tract, skin, and urinary tract infections have suggested that the peak/MIC ratio is the most important parameter (15). Although the results from these two studies appear to conflict, the importance of the peak/MIC ratio versus the AUC/MIC ratio may actually depend upon how high the peak/MIC ratio reaches (4). Nevertheless, these two parameters seem to be closely linked, and both have an important impact on the pharmacodynamics of fluoroquinolones and clinical outcome (8, 15).
Although it has been well established that the AUC/MIC ratio is an important pharmacodynamic parameter that affects the efficacies of fluoroquinolones, the optimum AUC/MIC ratio for different bacterial species and patient populations has been debated. On the basis of data from a study by Forrest and colleagues (8) with ciprofloxacin for the treatment of serious infections in hospitalized patients caused by gram-negative bacteria, a minimum AUC/MIC ratio of 125 has been suggested for all fluoroquinolones and bacterial pathogens. However, the pneumococcus appears to be unique in its interactions with fluoroquinolones. Clinical data with grepafloxacin against S. pneumoniae have suggested that AUC/MIC ratios below 125 are sufficient to achieve bacteriological eradication from patients with respiratory tract infections (7). Although the number of patients infected with S. pneumoniae was small in that study, a bacteriological eradication rate of 87.5% (seven of eight patients) was reported when AUC/MIC ratios were ≤92. Furthermore, pharmacodynamic studies with various fluoroquinolones against S. pneumoniae in IVPMs have shown that AUC/MIC ratios in the range of approximately 30 to 50 are sufficient to achieve eradication (9–11). However, those initial studies were not specifically designed to systematically evaluate the impacts of AUC/MIC ratios on the pharmacodynamics of fluoroquinolones. Therefore, the conclusions that can be reached from the data from those studies are limited. In order to truly understand the relationship between the AUC/MIC ratios of fluoroquinolones and pharmacodynamic interactions with S. pneumoniae, a systematic evaluation of the pharmacodynamics of fluoroquinolones against pneumococci over a range of AUC/MIC ratios is required.
In the present study, a two-compartment IVPM was used to expose strains of S. pneumoniae to a range of AUC/MIC ratios without altering the peak/MIC ratios. Because of the impact of peak/MIC ratios on the pharmacodynamics of fluoroquinolones discussed above, it was important to separate the AUC/MIC ratio from the peak/MIC ratio. Therefore, any differences in pharmacodynamics could be attributed more directly to the AUC/MIC ratios to which the bacteria were exposed. Furthermore, the bacterial strains were exposed to peak/MIC ratios of only 2:1 to 3:1. This low range of peak/MIC ratios was selected because some investigators have reported that the AUC/MIC ratio may be more important than the peak/MIC ratio when peak/MIC ratios are less than 10:1 (4). If a larger peak/MIC ratio had been used, the impacts of various AUC/MIC ratios may have been obscured by the influence of the peak/MIC ratio.
Targeting similar peak/MIC ratios in the present study resulted in comparable initial rates of killing of individual strains by gatifloxacin and levofloxacin. However, the achievement of comparable peak/MIC ratios did not specifically translate into comparable rates of killing of the different strains. Despite any differences in the initial rates of killing between strains, the overall impacts of the gatifloxacin and levofloxacin AUC/MIC ratios on eradication were similar. In general, when the AUC/MIC ratios were 27 to 34 or greater, eradication of all the strains was observed within 24 to 30 h. In contrast, when the AUC/MIC ratios were 17 to 24 or lower, eradication was not observed, and in many experiments viable counts increased rapidly to exceed the levels in the original inocula.
These data support the conclusions from previous studies with ciprofloxacin, ofloxacin, and levofloxacin that AUC/MIC ratios of approximately 30 to 50 are sufficient for the eradication of S. pneumoniae from IVPMs (9–11). However, data from the present study also suggest that once AUC/MIC ratios fall below 30, the likelihood of eradication is diminished. Although eradication of two strains was observed with AUC/MIC ratios as low as 27 and 28 (Fig. 1B and D), lack of eradication of the other two strains was observed, with AUC/MIC ratios of 22 and 24 (Fig. 1A and C). In contrast, eradication was observed in every experiment when AUC/MIC ratios were ≥30, including the comparative control experiments with levofloxacin.
These studies were designed to specifically evaluate the impacts of changing AUC/MIC ratios independent of the influence of peak/MIC ratios. However, despite the versatility of the IVPM used in the present study, it was not possible to separate T>MIC from the AUC/MIC ratio unless constant infusions were simulated, which would have required variations in peak/MIC ratios to achieve the desired range of AUC/MIC ratios. While T>MIC does not appear to play the most prominent a role in the pharmacodynamics of fluoroquinolones by statistical analysis, T>MIC undoubtedly can still affect pharmacodynamic interactions. In a study of levofloxacin for the treatment of respiratory tract, urinary tract, and skin infections, Preston and colleagues (15) found that T>MIC, the AUC/MIC ratio, and the peak/MIC ratio were indistinguishable in terms of their abilities to alter the probability of a successful outcome. Therefore, it was not surprising that some associations between T>MIC and eradication could be seen in the present study. In general, eradication of the S. pneumoniae strains from the IVPM required that gatifloxacin MICs remain above the MIC for longer than 8 h of the 24-h dosing interval. However, since there were limited data for T>MIC between 8 and 14 h, more specific conclusions could not be reached.
One final important observation from the present study was the lack of emergence of resistance, despite the simulation of peak levels that were only two- to threefold above the MIC for each strain. In contrast to the staphylococci, strains of S. pneumoniae have been comparatively slow in their development of fluoroquinolone resistance, despite the widespread use of ciprofloxacin since the late 1980s. Surveys of thousands of pneumococcal isolates from the United States between 1994 and 1997 yielded fluoroquinolone resistance rates of only 0.3 to 0.5% (2, 3). In 1997, a study of 2,284 clinical pneumococcal isolates from the United States, Europe, Latin America, and Canada yielded only 13 (0.6%) fluoroquinolone-resistant strains (14).
One possible explanation for the relative lack of fluoroquinolone resistance among pneumococci may be the restricted use of fluoroquinolones in pediatric patients, in whom long-term colonization with pneumococci presents an ideal environment for resistance selection. In addition, some data suggest that pneumococci lack the same propensity to mutate to develop the resistance that staphylococci and other species exhibit. In a recent study, Thomson and colleagues (K. S. Thomson, E. S. Moland, and C. C. Sanders, unpublished data) exposed cultures of 20 staphylococcal clinical isolates and 21 pneumococcal clinical isolates to ciprofloxacin and levofloxacin at concentrations two- to eightfold the MICs and evaluated the rate of selection of first-step mutants. In experiments with the staphylococci and ciprofloxacin, first-step mutants (exposed to drugs at more than fourfold the MICs) were isolated from 19 of 20 (95%) cultures of clinical isolates. In contrast, first-step mutants were selected from only 5 of 21 (24%) cultures of pneumococcal isolates exposed to ciprofloxacin and 3 of 21 isolates exposed to levofloxacin, even when concentrations were only twofold above the MIC. These data, in combination with the lack of resistance emergence in the present study, suggest that pneumococci do not exhibit the same propensity to mutate to resistance as seen with staphylococci and many gram-negative pathogens.
In summary, data from the present study support previous conclusions that AUC/MIC ratios of approximately 30 or higher are sufficient to achieve eradication of S. pneumoniae from IVPMs and that the impacts of AUC/MIC ratios on pharmacodynamics were similar among the strains, despite differences in susceptibilities and initial rates of bacterial killing. Furthermore, the data suggested that once the AUC/MIC ratios fall below 30, the likelihood of eradication is diminished. Further studies with strains with decreased susceptibilities to fluoroquinolones are needed to determine if the development of resistance affects the pharmacodynamic interactions between S. pneumoniae and fluoroquinolones. In addition, studies that evaluate the impacts of higher peak/MIC ratios (>10:1) on the relationship between AUC/MIC ratios and eradication would be beneficial to better delineate the linked importance of each of these two pharmacodynamic parameters for the treatment of pneumococcal infections.
Acknowledgments
This work was supported by a grant from Bristol-Myers Squibb.
I thank Jennifer Black for excellent technical assistance on this project.
REFERENCES
- 1.Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131–137. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann, A. B., K. C. Kugler, and G. V. Doern. 1997. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 41:1594–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doern, G. V., M. A. Pfaller, M. E. Erwin, A. B. Brueggemann, and R. N. Jones. 1998. The prevalence of fluoroquinolone resistance among clinically significant respiratory tract isolates of Streptococcus pneumoniae in the United States and Canada—1997 results from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 32:313–316. [DOI] [PubMed] [Google Scholar]
- 4.Drusano, G. L., D. E. Johnson, M. Rosen, and H. C. Standiford. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas aeruginosa sepsis. Antimicrob. Agents Chemother. 37:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley, M. N. 1991. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am. J. Med. 91(Suppl. 6A):45S–50S. [DOI] [PubMed] [Google Scholar]
- 6.Edberg, S. C. 1986. The meaurement of antibiotics in human fluids: techniques and significance, p. 381–476. In V. Lorian (ed.), Antibiotics in laboratory medicine, 2nd ed. The Williams & Wilkins Co., Baltimore, Md.
- 7.Forrest, A., S. Chodosh, M. A. Amantea, D. A. Collins, and J. J. Schentag. 1997. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute acterial exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 40(Suppl. A):45–57. [DOI] [PubMed] [Google Scholar]
- 8.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy, M. K., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 43:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:79–86. [DOI] [PubMed] [Google Scholar]
- 11.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 43:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lister, P. D., W. E. Sanders, Jr., and C. C. Sanders. 1998. Cefepime-aztreonam: a unique double β-lactam combination for Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Odland, B. A., R. N. Jones, J. Verhoef, A. Fluit, and M. L. Beach. 1999. Antimicrobial activity of gatifloxacin (AM-1155, CG5501) and four other fluoroquinolones tested against 2,284 recent clinical isolates Streptococcus pneumoniae from Europe, Latin America, Canada, and the United States. Diagn. Microbiol. Infect. Dis. 34:315–320. [DOI] [PubMed] [Google Scholar]
- 15.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornsief, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125–129 [DOI] [PubMed] [Google Scholar]
- 16.Schentag, J. J., D. E. Nix, and M. H. Adelman. 1991. Mathematical examination of dual individualization principles. I. Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP Ann. Pharmacother. 25:1050–1057. [DOI] [PubMed] [Google Scholar]
- 17.Smith, J. T. 1984. Awakening the slumbering potential of the 4-quinolone antibacterials. Pharm. J. 233:299–305. [Google Scholar]
- 18.Zabinski, R. A., A. J. Larsson, K. J. Walker, S. S. Gilliland, and J. C. Roschafer. 1993. Elimination of quinolone antibiotic carryover through use of antibiotic-removal beads. Antimicrob. Agents Chemother. 37:1377–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]