Abstract
The ability of entecavir (ETV) to inhibit Duck hepatitis B virus (DHBV) infection in duck hepatocytes and ducklings was examined using lamivudine (3TC) as a comparator drug. ETV exhibited antiviral activity (50% effective concentration [EC50], 0.13 nM) in DHBV-infected duck hepatocytes that was >1,000-fold more potent than that of 3TC (EC50, 138 nM). A 21-day treatment of ducklings with 1 mg of ETV per kg of body weight per day by oral gavage resulted in a mean reduction of log10 3.1 in serum DHBV DNA levels. Daily treatment with 0.1 mg of ETV/kg was nearly as effective, achieving an average viral DNA level decrease of log10 2.1. Reducing the daily dose of ETV to only 0.01 mg/kg resulted in an average viral DNA level decrease of log10 0.97. Daily treatment with 25 mg of 3TC/kg resulted in an average viral DNA level decrease of log10 0.66, compared to the log10 0.20 drop seen for ducklings given the vehicle alone. ETV was also more effective in decreasing the DHBV DNA levels in duck livers after 21 days of treatment, causing average drops of log10 1.41, log10 0.76, and log10 0.26 for dose levels of 1.0, 0.1, and 0.01 mg/kg, respectively, compared to a decrease of log10 0.06 for 3TC at a dose level of 25 mg/kg. Levels of viral covalently closed circular DNA in the treatment group receiving 1 mg of ETV/kg were reduced compared to those in the vehicle-treated group. ETV and 3TC were both well tolerated in all treated animals. These results show that ETV is a highly potent and effective antiviral in the DHBV duck model.
Hepatitis B virus (HBV), a small DNA virus that replicates through an RNA intermediate, is a leading cause of chronic hepatitis. The World Health Organization estimated in 1996 that 350 million people were persistently infected with the virus worldwide (8). Despite the availability of an effective vaccine against HBV, the prevalence of chronic infection has not significantly decreased (2). Persons with chronic hepatitis B not only suffer the wide range of symptoms associated with hepatitis but are at significant risk for the development of cirrhosis and/or primary hepatocellular carcinoma. Chronic carriers of HBV, moreover, constitute a reservoir for new infections.
Therapy currently consists of treatment with alpha interferon, which is associated with several undesirable side effects and variable, sometimes low, response rates (3), and treatment with lamivudine (3TC), a pyrimidine dideoxynucleoside (reviewed in reference 5). While effective in reducing viral load, 3TC treatment leads to resistance in both immunocompromised and immunocompetent patients with chronic HBV infections who receive the compound for extended periods (1, 6, 9). Consequently, there is an urgent need for new anti-HBV agents that are both safe and effective.
A number of compounds, most of which are nucleoside analogs that inhibit HBV polymerase and thereby interfere with replication, are currently under investigation for use in the chemotherapy of chronic HBV infection (for reviews, see references 3, 10, and 19). One of the most promising novel agents is entecavir (ETV; formerly BMS-200475), a guanosine analog that displays potent and selective inhibition of HBV. In HBV-producing HepG2 2.2.15 hepatoblastoma cell cultures, ETV exhibited potency in the nanomolar range, with a 50% effective concentration (EC50) of 0.00375 μM (7). ETV was also shown to be selective, since it had only modest activity against a panel of six unrelated RNA and DNA viruses (EC50s ranged from 10 to 80 μM) (7). Moreover, the concentration of ETV needed to cause 50% cytotoxicity (CC50) in HepG2 2.2.15 cell cultures was 30 μM, yielding a favorable selectivity index (CC50/EC50) of 8,000. Most importantly, ETV had no appreciable adverse effect on the mitochondrial DNA of proliferating HepG2 cells (7).
Studies of the mechanisms of action (14) confirmed that ETV triphosphate directly inhibits hepadnaviral polymerases and effectively suppresses the priming and elongation steps of HBV replication. To be effective antivirals, nucleoside analogs must be efficiently converted to their active triphosphate form. Phosphorylation studies examining ETV in both HepG2 and HBV-transfected HepG2 2.2.15 hepatoblastoma cells showed that ETV is readily phosphorylated by cellular enzymes to its triphosphate form with little accumulation of the intermediate mono- and diphosphate forms of ETV (18). In addition, the intracellular half-life was determined to be relatively long (15 h) (18).
Previous in vivo studies utilizing oral treatment of woodchucks (Marmota monax) chronically infected with Woodchuck hepatitis virus (WHV) have demonstrated the potency and effectiveness of ETV in reducing viral DNA concentrations to undetectable levels after daily administration of 0.02, 0.1, or 0.5 mg/kg of body weight for a period of 3 months (4). ETV was well tolerated, with no evidence of toxicity during the treatment or the 3-month posttreatment follow-up period.
The Duck hepatitis B virus (DHBV)-infected Pekin duck (Anas platyrhynchos) is also widely accepted as a model for evaluating new agents directed against HBV. The replication cycles of DHBV, WHV, and HBV are essentially the same (for reviews, see references 10 and 19). The purpose of the present study was to evaluate the ability of ETV to inhibit hepadnavirus replication in this alternative HBV animal model by measuring levels of DHBV DNA in infected primary duck hepatocyte cultures and in DHBV-infected Pekin ducklings. In the in vivo evaluation, ducklings received ETV orally in three different dosages, a control group received the vehicle (distilled water) as a placebo, and a reference group received 3TC in a single dosage.
MATERIALS AND METHODS
In vitro evaluation. (i) Cell culture.
Primary duck hepatocytes were prepared from 10-day-old LeGarth or Berlill hybrid Pekin ducks by a modification of the method of Tuttleman et al. (16). Cells were seeded into 48-well plates at 2.25 × 105 cells per well and were cultured overnight in L-15 medium plus 5% fetal calf serum. The medium was then changed to L-15 plus 1.5% dimethyl sulfoxide. This medium was used for all subsequent culturing of the cells. For evaluation of cytotoxicity, cells were seeded into low-evaporation, 96-well plates at 4.5 × 104 cells per well and were cultured as described above.
(ii) Assay for virus replication.
Monolayers of primary duck hepatocytes in 48-well plates were infected with pooled inoculum derived from ducklings congenitally infected with DHBV16 (11) at a multiplicity of infection of 100 50% duck infectious doses per cell on day 2 after plating. Treatment with the study drugs lasted from day 4 after seeding through day 16 at a series of concentrations designed to generate information concerning the EC50 of each drug. The medium was changed every day until day 6 after seeding and every 3 days thereafter. The inhibitors were made up in culture medium weekly and stored at 4°C until use. The cells were collected by trypsinization on day 12 of treatment. Intracellular viral DNA levels were evaluated by modified slot blot DNA hybridization similar to that described previously (12) by using a Molecular Imager (Bio-Rad) for detection and for quantification relative to a curve derived from blotted, known amounts of DHBV DNA. With this assay, the level of detection was 10 pg of DHBV DNA/well.
(iii) Assay for cytotoxicity.
Cytotoxicity was evaluated using an XTT assay described previously (17). Cells in 96-well plates were exposed to the drugs at the same concentrations used for the determination of the EC50s. After 7 days of drug treatment, the medium was removed from each well and replaced with 200 μl of medium (phenol red minus RPMI medium plus 10% fetal calf serum) containing 0.3 mg of XTT tetrazolium (Polysciences, Inc., Warrington, Pa.)/ml and 0.01 mg of phenazine methosulfate (Sigma-Aldrich, Inc., St. Louis, Mo.)/ml. After incubation at 37°C for 4 h, the optical densities at 450 nm were read for all wells in an enzyme-linked immunosorbent assay (ELISA) plate reader. The CC50 was defined as the drug concentration required to limit cell monolayers to 50% of the XTT tetrazolium-derived color intensity of untreated wells.
In vivo evaluation. (i) Experimental animals.
Thirty ducklings were used in the in vivo drug evaluation; 28 of them were LeGarth Pekin ducklings obtained the day after they hatched from Metzer Farms, Gonzales, Calif., and 2 of them were ducklings congenitally infected with the Pekino strain of DHBV (P. L. Marion, unpublished data) that had hatched at the same time as the LeGarth ducklings. Two of the LeGarth ducklings were congenitally infected with DHBV. The other 26 were injected at 3 days of age with 105.5 50% duck infectious doses of the DHBV16 pool used in the in vitro evaluation. All ducklings were housed in the laboratory animal facility of the Stanford University Medical Center, Stanford, Calif. The experiments were reviewed and approved by the Stanford University Institutional Animal Care and Use Committee.
(ii) Experimental plan.
Three-week-old, DHBV-infected ducklings were treated in groups of six, as shown in Table 1. All treatments were delivered by gavage. The antiviral activity of the inhibitors was assessed by comparing the serum DHBV DNA levels of inhibitor-treated and control ducklings before initiation of treatment, during a 3-week course of therapy, and during a posttreatment follow-up period of 2 weeks. On days −8 and −4, blood samples were taken, and sera were prepared for analysis of DHBV DNA levels. On day 0, blood samples were taken and the ducklings were weighed and treated with a drug or the placebo. Treatment continued for 3 weeks, during which time the animals were weighed daily. Blood samples were taken on days 4, 7, 12, 16, and 21. On day 21, three of the ducklings in each group were sacrificed, and their livers were removed and stored frozen for subsequent analysis of viral DNA. The remaining animals were monitored for 2 weeks after cessation of treatment, with blood samples taken on days 25, 30, and 35. On day 35, these ducklings were sacrificed, and their livers were collected for quantification of viral DNA.
TABLE 1.
Baseline serum DHBV titers for treatment groups
| Drug, dose, and duckling no. | DHBV titer (pg/ml)a |
|---|---|
| ETV, 1 mg/kg | |
| 126 | 15,273 |
| 133 | 9,447 |
| 139 | 41,742 |
| 154 | 23,058 |
| 161 | 45,961 |
| 204b | 67,045 |
| ETV, 0.1 mg/kg | |
| 127 | 11,767 |
| 129 | 15,811 |
| 145 | 26,953 |
| 221 | 44,897 |
| 229 | 41,284 |
| 273b | 64,914 |
| ETV, 0.01 mg/kg | |
| 132 | 17,473 |
| 143 | 27,390 |
| 151 | 43,399 |
| 153 | 38,590 |
| 155 | 12,098 |
| 158 | 54,316 |
| 3TC, 25 mg/kg | |
| 148 | 30,406 |
| 157 | 35,567 |
| 247 | 49,264 |
| 281 | 13,079 |
| 283b | 42,812 |
| 299 | 21,966 |
| None (control) | |
| 136 | 33,514 |
| 142 | 46,174 |
| 150 | 14,775 |
| 242b | 41,098 |
| 246 | 31,521 |
| 278 | 22,159 |
Baseline titers were performed on day −4.
Duckling that was congenitally infected with DHBV.
(iii) Slot blot assay for DHBV DNA in sera and livers.
Total DHBV DNA levels in sera and in liver DNA extracts were evaluated by the modified slot blot DNA assay described for the in vitro evaluation, with sensitivities of 100 pg of DHBV DNA per ml of serum and 1 pg of DHBV DNA per μg of liver DNA. Covalently closed circular DNA (cccDNA) was purified from liver homogenates as described previously (15) and was analyzed by electrophoresis through 1% agarose gels followed by blotting onto nylon membranes (Hybond N; Amersham Pharmacia Biotech, Piscataway, N.J.). Hybridization was performed as described previously (12) with a HiPrime Random Primer kit (Roche Diagnostics Corporation, Indianapolis, Ind.) for radiolabeling and DHBV16 DNA as a template.
(iv) PCR assay for DHBV DNA in sera.
When viral titers were too low to be easily detected by slot blot hybridization, a quantitative DHBV DNA PCR ELISA was used. This assay is parallel to one created for HBV DNA (P. L. Marion and M. A. Winters, unpublished data) that gives results equivalent to those obtained with the Amplicor system (Roche Diagnostics) (regression coefficient, 0.857; sample number, 71; P < 0.001). The same DHBV DNA standard was used for both the slot blot and PCR assays, and PCR tests on slot blot-positive samples provided similar quantitative results. The lower limit of quantification in this assay is 10 DHBV copies. The mean variability ranges from 1.5-fold in the same assay to 1.8-fold in samples tested in different assays. Standard procedures for minimizing PCR contamination (e.g., geographic separation of sample preparation, performance of pre-PCR steps, use of barrier tips during pipetting, and inclusion of randomly placed negative controls) were employed. Duckling serum was diluted in phosphate-buffered saline containing 10% fetal bovine serum and denatured with an equal volume of 0.2 N sodium hydroxide. After incubation at 60°C, the mixture was neutralized and added to a PCR master mixture containing a buffer (2.5 mM magnesium chloride, 200 μM deoxynucleoside triphosphate, 2.5 U of Taq DNA polymerase [Life Technologies, Inc., GIBCO/BRL, Grand Island, N.Y.]) and 30 pmol each of primers DHBV-1 (TACTAGCTGGCCTAATCGGA) and DHBV-2 (GGCAGTAGTGAAGAGATGGA) (13). DHBV-2 was biotinylated. Serial 0.5 log dilutions of DHBV-containing plasmid were amplified in parallel, along with duck serum standards prepared in-house. The PCR product was then quantified after being subjected to binding to avidin-coated plates and probing with a digoxigenin-labeled DHBV oligonucleotide (GGAGGCTAGACTGGTGGTGGAT); this oligonucleotide was detected by a peroxidase-labeled antidigoxigenin antibody (Boehringer Mannheim Corporation, Indianapolis, Ind.) that had reacted with an appropriate substrate. Optical densities were read against a curve generated by using known amounts of DHBV DNA.
RESULTS
Antiviral activity in DHBV-infected primary duck hepatocyte cultures.
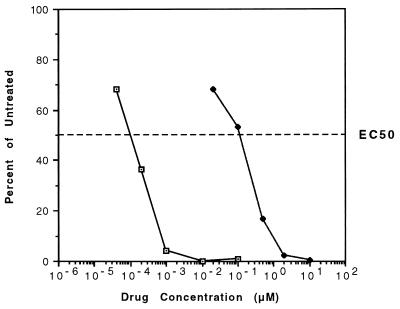
ETV was first evaluated for efficacy against DHBV in cultured monolayers of primary duck hepatocytes. Quantitation by slot blot titration of intracellular DHBV DNA levels after 12 days of treatment with serial dilutions of ETV and 3TC yielded EC50s of 0.00013 and 0.14 μM, respectively, indicating that ETV is about 1,000-fold more potent than 3TC against this virus (Fig. 1). The CC50 for ETV was 8.1 μM, yielding a very favorable selectivity index of 62,300. No cellular toxicity was seen with the highest levels of 3TC tested (100 μM or 714-fold higher than its EC50). These results confirmed the potent inhibitory effect of ETV against the hepadnavirus family and indicated that DHBV may be exquisitely sensitive to the drug.
FIG. 1.
Inhibitory effect of ETV (□) and 3TC (⧫) on the replication of DHBV in primary hepatocyte cultures. The curves represent intracellular DHBV DNA levels as percentages of those for untreated controls after 12 days of drug treatment. The values are means of results of at least three independent assays. Standard deviations of the EC50 values were ±0.00006 μM for ETV and ±0.12 μM for 3TC.
Evaluation of DHBV-infected Pekin duckling model.
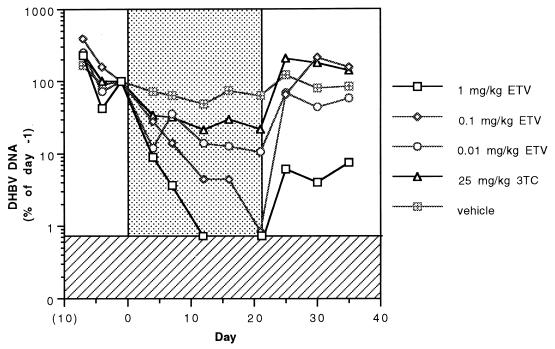
Figure 2 shows the mean concentrations of DHBV DNA in the sera of ducklings as determined by slot blot assay and expressed as percentages of baseline (day −1) values before, during, and after daily treatment with the vehicle, with 3TC, or with three different dose levels of ETV. The results clearly show that the anti-DHBV activity of ETV in the duckling model is dose dependent and that even the lowest daily ETV dose of 0.01 mg/kg is more effective than 3TC administered at a dose of 25 mg/kg per day. After 12 days of treatment with 1 mg of ETV/kg, mean viral concentrations dropped below the level of detection and were considerably below the pretreatment level.
FIG. 2.
Mean concentrations of DHBV DNA in the sera of ducklings before, during (days 1 to 21, n = 6 in each treatment group), and after (days 22 to 35, n = 3 in each group) treatment with the vehicle (distilled water) or the indicated daily dose of 3TC or ETV. The stippled area of the graph indicates the treatment period, and the hatched area shows the limit of detection by slot blotting. Evaluations of statistical significance were performed by the Student t test for day-21 concentrations compared to those for vehicle-treated controls at a P of <0.01 for all three doses of ETV and a P of 0.089 for 25 mg of 3TC/kg.
The serum DHBV DNA levels in the individual vehicle-treated ducklings remained relatively constant over the 21 days of treatment, although the levels in four of the six animals showed gradual declines (data not shown). The mean reduction in serum viral DNA levels in the control (i.e., vehicle-treated) group during the 21-day treatment period was 51% (log10 0.31). All of the animals in the 3TC-treated group showed a reduction (mean, 78% or log10 0.66) in DHBV DNA levels over the course of treatment. Viremia rapidly returned to pretreatment levels in all three ducks in the 3TC-treated group that were monitored during the 2-week posttreatment period. A rise in viral DNA levels above pretreatment levels was noted in all of these ducks after treatment.
The ETV-treated ducklings showed a marked decrease in serum DHBV DNA levels for all three dose levels. Treatment with 1 mg of ETV/kg per day resulted in a drop in DHBV DNA to undetectable levels (by slot blotting) in all six ducklings by day 12, and only one of the three ducklings monitored during the posttreatment period showed a rebound in its viral titer (data not shown). Five of the six ducklings treated with daily doses of 0.1 mg of ETV/kg showed viral DNA reductions (mean, >99% or log10 2.1) to undetectable slot blot levels by day 21. All three of the animals observed posttreatment showed a rebound to pretreatment concentrations. For the ducklings treated with 0.01 mg of ETV/kg per day, DHBV DNA levels also decreased, but the mean decrease (90%) was somewhat lower than those with the higher doses, and only one of the treated animals achieved undetectable DNA slot blot levels.
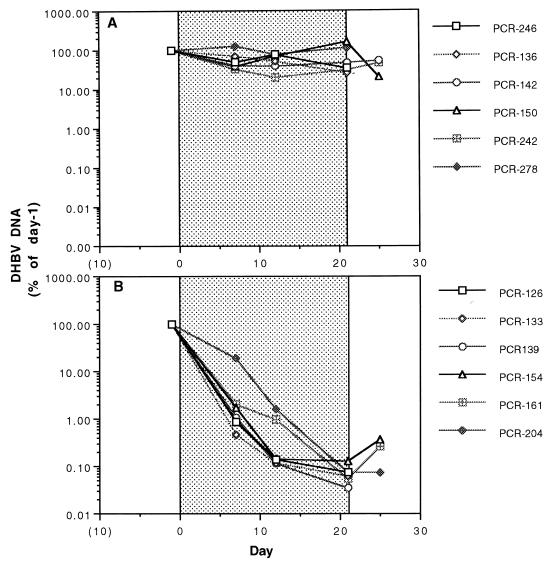
A quantitative DHBV DNA PCR ELISA was developed to increase the sensitivity of detection to a level below that allowed by slot blotting. The results of the PCR-based assay done on sera from ducklings treated with the placebo and sera from those treated with 1.0 mg of ETV/kg per day can be seen in Fig. 3A and B, respectively. Using this assay, the mean reduction in serum DHBV DNA with the highest dose of ETV was log10 3.12 (range, log10 2.76 to log10 3.46) after 21 days of treatment, whereas that of the placebo-treated group was log10 0.27.
FIG. 3.
Serum DHBV DNA concentrations determined by a quantitative PCR ELISA in individual vehicle-treated ducklings (A) and in ducklings treated with 1 mg of ETV/kg per day for 21 days (B). The stippled areas of the graphs indicate the treatment periods. Evaluations of statistical significance were performed by the Student t test for day-21 concentrations compared to those for vehicle-treated controls, yielding a P of <0.025.
Viral DNA levels in liver homogenates.
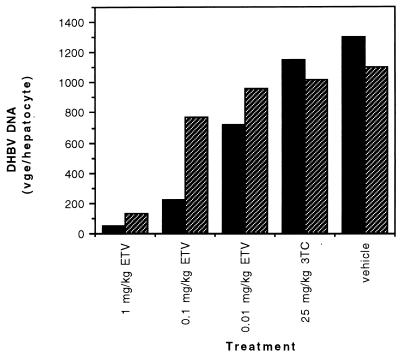
Because the liver is the predominant target organ of hepadnavirus infection, DHBV DNA concentrations in the liver homogenates of the individual ducklings at the end of the treatment and posttreatment periods were determined (Fig. 4). The ducklings in the 3TC-treated group exhibited mean viral DNA levels that were 88 and 93% of the level in the control group on days 21 and 35, respectively. All three ETV dose levels again showed superior activity. At day 21, the animals in the groups receiving daily ETV doses of 1, 0.1, and 0.01 mg/kg showed mean DHBV DNA concentrations that were 4, 17, and 55% of the control group level, respectively. At day 35, the mean viral DNA levels in the three ETV-treated groups rose to 12, 70, and 87% of the control group level, respectively.
FIG. 4.
Mean DHBV DNA concentrations in the livers of ducklings (n = 3 in each treatment group) at the end of treatment (day 21; solid bars) and 2 weeks after the end of treatment (day 35; hatched bars) with the vehicle (distilled water) or the indicated daily dose of 3TC or ETV. DHBV DNA is expressed in viral genome equivalents (vge) per hepatocyte. For this calculation, the following equivalencies were used: 1 copy of DHBV genome was equivalent to 3 × 10−6 pg, and 1 hepatocyte contained 5 × 10−6 μg of DNA.
Reduction in viral cccDNA levels.
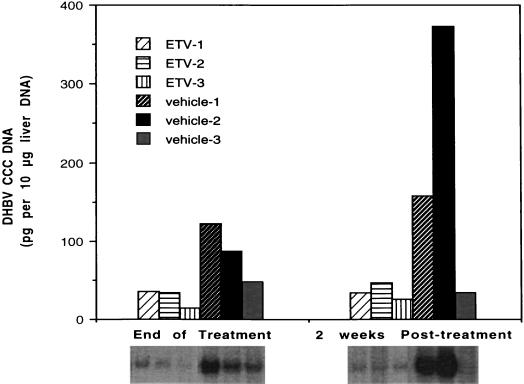
Chronic HBV infections in humans are believed to depend on the maintenance of a key replicative intermediate known as cccDNA. Viral cccDNA is the key DNA replicative form used as a template for transcription of viral components, including the pregenomic RNA that serves as a template for virion DNA. Levels of cccDNA were determined in the ducklings treated with the placebo and in those treated with 1.0 mg of ETV/kg after 21 days of treatment and at the end of the 2-week follow-up period (Fig. 5). Although the levels of this viral marker varied from duckling to duckling in the placebo-treated group, a mean threefold reduction in the level of cccDNA was seen in the ETV-treated ducklings relative to the control level at the end of the 3-week treatment period. These reduced levels were maintained during the 2-week posttreatment period, when the ETV-treated ducklings showed cccDNA concentrations that were more than fivefold lower than the control level.
FIG. 5.
Levels of DHBV cccDNA in individual ducklings after treatment with daily doses of the vehicle (distilled water) or 1.0 mg of ETV/kg at day 21 and at the end of the 2-week follow-up period. ETV-1, -2, and -3 and vehicle-1, -2, and -3 are designations of individual ducks treated with either ETV or vehicle. Note that ducks analyzed at the end of treatment are not the same ducks as those analyzed posttreatment, although labels are the same.
Treatment was well tolerated at all dose levels.
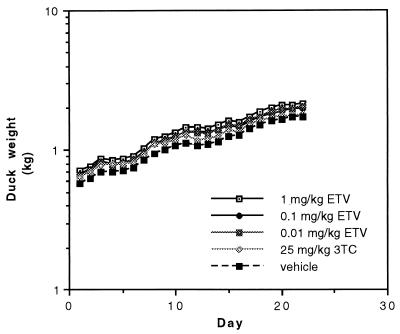
The tolerability of the inhibitors was assessed by monitoring weight gain and the general appearance of the ducklings in the six groups during treatment. There were no significant differences in mean weight gain among the treatment groups (Fig. 6), which suggested that ETV and 3TC, at the doses used and in this limited study, were not toxic to the growing ducklings. While the livers of ETV- and 3TC-treated ducklings appeared indistinguishable from those of vehicle-treated (i.e., placebo-treated) ducklings at necropsy, a detailed toxicity study involving the monitoring of blood chemistry was not done. All of the animals appeared to be in good health throughout the study, except for a vehicle-treated duckling that inexplicably developed a swollen hock on study day 2. The duckling was kept in the study because its viral titers did not differ from those of the other animals in its group.
FIG. 6.
Weight development of the ducklings during treatment (n = 6 in each group) with the vehicle (distilled water) or the indicated daily dose of 3TC or ETV.
DISCUSSION
Chronic HBV infection remains a serious and life-threatening illness for millions worldwide. The frequent emergence of 3TC-resistant variants during 3TC treatment underscores the need for more effective therapies. In vitro and in vivo studies of ETV have repeatedly demonstrated its excellent potency, selectivity, and overall potential to be an effective inhibitor of HBV infection. The present studies were performed with the duck model to further characterize the antiviral activity of ETV against the high-titer hepadnavirus infection of a natural host.
In initial studies, determination of intracellular DHBV levels in duck primary hepatocyte cultures after treatment with serial dilutions of ETV showed this nucleoside analog to be a highly active (EC50, 0.13 nM) and selective (selectivity index, >62,000) inhibitor of DHBV replication. It is difficult to compare this measured level of potency to those published, since there is variation among laboratory assay systems. Nevertheless, the efficacy of ETV against DHBV appears to equal, if not exceed, published potency levels of all other nucleoside analogs effective against this virus (3, 10). ETV was clearly superior to over 30 compounds with known anti-HBV activity that were tested in the cell culture system described here (P. Marion and R. Schinazi, unpublished data). Initial results from studies involving a variety of combinations of both ETV and 3TC in cell culture suggest that combinations of these two antivirals are likely to be additive (unpublished data).
In ducklings, the anti-DHBV activity of ETV was dose dependent, with all three daily dosage levels (0.01, 0.1, and 1 mg/kg) being considerably more effective than 25 mg of 3TC/kg per day in inhibiting DHBV replication. It should be noted that a single daily oral delivery of this dosage of 3TC was not an optimal treatment regimen for this virus-host model. The 2,500-fold-greater efficacy of ETV in ducks (Fig. 2) surpasses the differences determined in cell culture assays and suggests that differences in intracellular triphosphate levels, differences in pharmacokinetic parameters, or other factors may contribute to the enhanced potency observed in treated ducks. Interestingly, this multilog reduction in DHBV viremia after 7 days of treatment with 1 mg of ETV/kg per day (Fig. 3B) was very similar to that noted in a previous study of WHV-infected woodchucks treated with 0.1 mg of ETV/kg per day (4).
To be effective, anti-HBV drugs must be able to reduce or eliminate viral infection within the liver. Decreases in viral levels in the liver, as judged by measurement of viral DNA in liver samples, mimicked the reductions observed in serum samples, though the overall reduction was less in the liver. Ducklings treated with the highest dose of ETV experienced the greatest reduction compared to those treated with the control (Fig. 4). One of the most difficult HBV DNA targets to reduce in amount or to eliminate is the key replicative intermediate, cccDNA. This viral DNA form is the most resistant to antiviral treatment, since it does not replicate and is not thought to be directly sensitive to nucleoside analog inhibitors. During the relatively short 21-day treatment period, ETV therapy did reduce the levels of DHBV cccDNA by threefold compared with that of the controls. This relative reduction of cccDNA levels in ducklings that were still growing is most likely due to the inhibition of infection of new hepatocytes along with a reduction in the rate of recycling of mature viral DNA into the nucleus during the usual amplification of cccDNA in infected hepatocytes.
At the end of 3 weeks of daily treatment with 1 mg of ETV/kg, a delay in viral rebound was noted in samples of both the serum and the liver, in contrast to the increases in viral titers in serum to levels equal to or exceeding pretreatment levels for ducks treated with 25 mg of 3TC/kg. While the factors resulting in the delay in viral rebound in ducks treated with the highest ETV dose have not yet been determined experimentally, both the significant inhibition of DHBV DNA levels achieved and the ability to limit cccDNA levels may contribute to the sustained posttreatment response. In summary, these results, and the fact that ETV was well tolerated by the animals in our study, further support the continued clinical development of ETV for the treatment of chronic HBV infection in humans.
Acknowledgments
This work was supported by a grant from the Pharmaceutical Research Institute, Bristol-Myers Squibb, Wallingford, Conn.
REFERENCES
- 1.Bartholomew, M. M., R. W. Jansen, L. J. Jeffers, K. R. Reddy, L. C. Johnson, H. Bunzendahl, L. D. Condreay, A. G. Tzakis, E. R. Schiff, and N. A. Brown. 1997. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet 349:20–22. [DOI] [PubMed] [Google Scholar]
- 2.Colacino, J. M., and K. A. Staschke. 1998. The identification and development of antiviral agents for the treatment of chronic hepatitis B virus infection. Prog. Drug Res. 50:259–322. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq, E. 1999. Perspectives for the treatment of hepatitis B virus infections. Int. J. Antimicrob. Agents 12:81–95. [DOI] [PubMed] [Google Scholar]
- 4.Genovesi, E. V., L. Lamb, I. Medina, D. Taylor, M. Seifer, S. Innaimo, R. J. Colonno, D. N. Standring, and J. M. Clark. 1998. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob. Agents Chemother. 42:3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagmeyer, K. O., and Y. Y. Pan. 1999. Role of lamivudine in the treatment of chronic hepatitis B virus infection. Ann. Pharmacother. 33:1104–1112. [DOI] [PubMed] [Google Scholar]
- 6.Honkoop, P., H. G. Niesters, R. A. de Man, A. D. Osterhaus, and S. W. Schalm. 1997. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J. Hepatol. 26:1393–1395. [DOI] [PubMed] [Google Scholar]
- 7.Innaimo, S. F., M. Seifer, G. S. Bisacchi, D. N. Standring, R. Zahler, and R. J. Colonno. 1997. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob. Agents Chemother. 41:1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS. 1998. Report on the global HIV/AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
- 9.Ling, R., D. Mutimer, M. Ahmed, E. H. Boxall, E. Elias, G. M. Dusheiko, and T. J. Harrison. 1996. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology 24:711–713. [DOI] [PubMed] [Google Scholar]
- 10.Luscombe, C. A., and S. A. Locarnini. 1996. The mechanism of action of antiviral agents in chronic hepatitis B. Viral Hepatitis Rev. 2:1–35. [Google Scholar]
- 11.Mandart, E., A. Kay, and F. Galibert. 1984. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J. Virol. 49:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marion, P. L., J. M. Cullen, R. R. Azcárraga, M. J. Van Davelaar, and W. S. Robinson. 1987. Experimental transmission of duck hepatitis B virus to Pekin ducks and to domestic geese. Hepatology 7:724–731. [DOI] [PubMed] [Google Scholar]
- 13.Schroder, I., B. Holmgren, M. Oberg, and B. Lofgren. 1998. Inhibition of human and duck hepatitis B virus by 2′,3′-dideoxy-3′-fluoroguanosine in vitro. Antivir. Res. 37:57–66. [DOI] [PubMed] [Google Scholar]
- 14.Seifer, M., R. K. Hamatake, R. J. Colonno, and D. N. Standring. 1998. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob. Agents Chemother. 42:3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summers, J., P. M. Smith, and A. L. Horwich. 1990. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J. Virol. 64:2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuttleman, J. S., J. C. Pugh, and J. W. Summers. 1986. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J. Virol. 58:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weislow, O. S., R. Kiser, D. L. Fine, J. Bader, R. H. Shoemaker, and M. R. Boyd. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 81:577–586. [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka, G., T. Wilson, S. Innaimo, G. S. Bisacchi, P. Egli, J. K. Rinehart, R. Zahler, and R. J. Colonno. 1999. Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob. Agents Chemother. 43:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoulim, F., and C. Trépo. 1999. New antiviral agents for the therapy of chronic hepatitis B virus infection. Intervirology 42:125–144. [DOI] [PubMed] [Google Scholar]