Abstract
The therapeutic efficacy of BAL9141 (formerly Ro 63-9141), a novel cephalosporin with broad in vitro activity that also has activity against methicillin-resistant Staphylococcus aureus (MRSA), was investigated in rats with experimental endocarditis. The test organisms were homogeneously methicillin-resistant S. aureus strain COL transformed with the penicillinase-encoding plasmid pI524 (COL Bla+) and homogeneously methicillin-resistant, penicillinase-producing isolate P8-Hom, selected by serial exposure of parent strain P8 to methicillin. The MICs of BAL9141 for these organisms (2 mg/liter) were low, and BAL9141was bactericidal in time-kill curve studies after 24 h of exposure to either two, four, or eight times the MIC. Rats with experimental endocarditis were treated in a three-arm study with a continuous infusion of BAL5788 (formerly Ro 65-5788), a carbamate prodrug of BAL9141, or with amoxicillin-clavulanate or vancomycin. The rats were administered BAL9141 to obtain steady-state target levels of 20, 10, and 5 mg of per liter or were administered either 1.2 g of amoxicillin-clavulanate (ratio 5:1) every 6 h or 1 g of vancomycin every 12 h at changing flow rates to simulate the pharmacokinetics produced in humans by intermittent intravenous treatment. Treatment was started 12 h after bacterial challenge and lasted for 3 days. BAL9141 was successful in the treatment of experimental endocarditis due to either MRSA isolate COL Bla+ or MRSA isolate P8-Hom at the three targeted steady-state concentrations and sterilized >90% of cardiac vegetations (P < 0.005 versus controls; P < 0.05 versus amoxicillin-clavulanate and vancomycin treatment groups). These promising in vivo results with BAL9141 correlated with the high affinity of the drug for PBP 2a and its stability to penicillinase hydrolysis observed in vitro.
Over the years methicillin-resistant Staphylococcus aureus (MRSA) has acquired stable resistance against almost all clinically available antibiotics, including aminoglycosides, macrolides, sulfamethoxazole-trimethoprim, tetracyclines, rifampin, and fluoroquinolones. Thus, therapeutic options for MRSA infections are scarce or are limited to glycopeptide-type drugs such as vancomycin or, more recently, quinupristin-dalfopristin or linezolid (16). However, clinical isolates of S. aureus with reduced susceptibilities to glycopeptides, known as glycopeptide-intermediate S. aureus or vancomycin-intermediate S. aureus, have recently been described in Japan, the United States, and Europe (14, 17, 19). Therefore, MRSA isolates present a major challenge in terms of chemotherapy because effective antimicrobial treatment options for infections caused by these isolates are close to becoming exhausted.
Most MRSA isolates resist virtually all β-lactam antibacterials by two mechanisms: (i) production of penicillinase and (ii) production of a new, low-affinity penicillin-binding protein (PBP) called PBP 2a. Several studies have revealed that certain “time-honored” β-lactams molecules such as penicillin G, ampicillin, and amoxicillin with relatively good affinities for PBP 2a have demonstrable anti-MRSA activities in vitro and in animals with experimental endocarditis due to penicillinase-negative isolates (2, 8, 10, 15). However, successful treatment of infections caused by penicillinase-producing isolates required the addition of critical amounts of β-lactamase inhibitors, such as sulbactam or clavulanate, to protect the β-lactams from staphylococcal penicillinase-induced hydrolysis (8, 10, 15). Likewise, studies with animals confirmed that PBP 2a can be effectively blocked in vivo by β-lactams with improved affinities for PBP 2a. Thus, β-lactams must combine both a high affinity for PBP 2a and stability against degradation by staphylococcal penicillinase to be considered for clinical use against infections caused by MRSA.
BAL9141 (formerly Ro 63-9141), a novel parenteral cephalosporin which has been designed to possess the latter set of properties described above, meets these requirements against gram-positive bacteria including MRSA (12). The good activity of this compound stems from its potent inhibition of PBP 2a combined with its high degree of resistance to attack by β-lactamases (12).
The aim of the present study was to investigate the relevance of these properties of BAL9141 in vivo. To this end, the therapeutic efficacy of BAL9141 was investigated in rats with experimental endocarditis due to penicillinase-producing MRSA expressing homogeneous resistance to methicillin. Amoxicillin-clavulanate and vancomycin were used as reference drugs.
MATERIALS AND METHODS
Microorganisms and growth conditions.
Two isolates of MRSA expressing homogeneous resistance to methicillin were used in the animal studies: (i) a penicillinase-producing transformant of strain COL, strain COL Bla+, obtained by DNA transformation with penicillinase-encoding plasmid pI524 (18), and (ii) a homogeneously methicillin-resistant, penicillinase-producing strain, strain P8-Hom, selected by serial passage of parent strain P8 on methicillin-containing medium as described previously (20). Strain P8-Hom was stable, as it appeared to retain its phenotype of homogeneous resistance for up to 60 generations in the absence of antibiotic pressure in vitro. In certain experiments penicillinase-negative parent strain COL (COL Bla−) was also used. Unless stated otherwise the bacteria were grown at 35°C in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with aeration or on tryptic soy agar (Difco) supplemented with either 2% NaCl (for methicillin) or penicillinase (final concentration, 2,000 U/ml; Bacto-Penase concentrate; Difco) (penicillinase was added for amoxicillin-clavulanate as a precaution to avoid antibiotic carryover). Stocks were kept at −70°C in tryptic soy broth supplemented with 10% (vol/vol) glycerol.
Antibiotics.
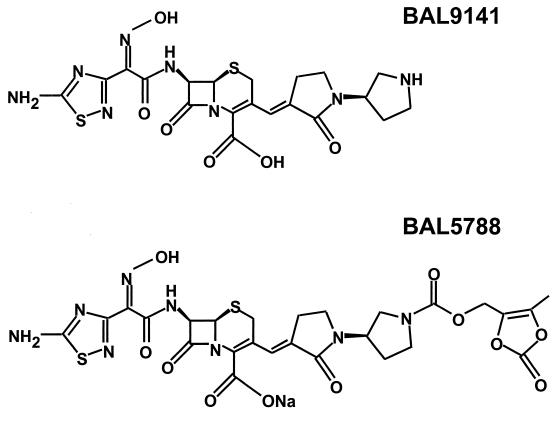
BAL9141 and BAL5788 (formerly Ro 65-5788), the prodrug of BAL9141, both of known potency, were supplied by F. Hoffmann-La Roche AG (Basel, Switzerland); methicillin, amoxicillin, and amoxicillin-clavulanate (5:1 [wt/wt] ratio) were purchased from SmithKline Beecham AG (Thörishaus, Switzerland); vancomycin was obtained from Eli Lilly (Vernier, Switzerland). The chemical structures of BAL9141 and its prodrug, BAL5788, are shown in Fig. 1.
FIG. 1.
Chemical structures of BAL9141 and its prodrug, BAL5788.
Population analysis profile.
The phenotypic expression of β-lactam resistance was performed by spreading a large bacterial inoculum (109 CFU) as well as smaller inocula (106, 105, and 103 CFU) onto tryptic soy agar plates containing twofold dilutions of antibiotics (10). The numbers of colonies growing on the plates were enumerated after 48 h of incubation at 35°C. Population analysis profile curves were generated by plotting the numbers of colonies growing on the plates against the concentrations of antibiotics in the plates.
MIC determinations.
The MICs of the antibiotics were determined in Mueller-Hinton broth (MHB; Difco) (for methicillin, the MHB was supplemented with 2% NaCl) with an inoculum size of 106 CFU. The MIC was defined as the lowest antibiotic concentration that inhibited visible bacterial growth after 24 h of incubation at 35°C.
Time-kill curves.
The bactericidal activities of BAL9141, amoxicillin-clavulanate, and vancomycin were measured by time-kill curves. Series of flasks containing fresh prewarmed medium (MHB) were inoculated with approximately 106 CFU of bacteria from an overnight culture. Immediately after inoculation the antibiotics were added to the flasks at final concentrations approximating the levels expected in the serum of humans after administration of therapeutic doses of the compounds (see Results). These concentrations were 20, 10, and 5 mg/liter for BAL9141; 90 mg of amoxicillin per liter and 20 mg of clavulanate per liter for amoxicillin-clavulanate, and 40 mg/liter for vancomycin. Viable counts were determined just before and at various times after the addition of antibiotics by plating adequate dilutions of the cultures on agar plates. To avoid antibiotic carryover, 0.5-ml samples of the cultures were transferred from the flasks into microcentrifuge tubes, and the bacteria were pelleted and resuspended twice in antibiotic-free medium to remove residual drugs. Bacterial suspensions were then serially diluted and plated as described above. Each time-kill experiment was performed on two independent occasions.
Binding affinities of BAL9141 for PBP 2a.
The presence of PBP 2a was determined in membrane fractions of bacterial lysates of strains COL Bla+ and P8-Hom, as described previously (10). The binding affinities of BAL9141 for PBP 2a were assessed by measuring its ability to compete with binding of [3H]penicillin to PBPs, as described previously (10, 18). The binding affinities of BAL9141 were derived from densitometric quantifications and were expressed as the drug concentration that inhibited binding of [3H]penicillin by 50% (IC50).
Penicillinase stability of BAL9141.
The abilities of BAL9141 and amoxicillin to resist hydrolysis by penicillinase following exposure to a large inoculum (109 CFU) of strains COL Bla+ and P8-Hom were measured in broth cultures by a bioassay (11, 18).
Production of experimental endocarditis and infusion pump installation.
The production of catheter-induced aortic vegetations in the rats and the installation of a programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, Mass.) for delivery of the antibiotics were performed while the rats were under general anesthesia, as described previously (9, 13). Less than 10% of the rats died as a result of either the anesthesia or the catheter-induced cardiac arrhythmia. Infectious endocarditis was induced 24 h after placement of the aortic catheter by intravenous (i.v.) challenge of the animals with 0.5 ml of saline containing 105 CFU of either of the test organisms. This inoculum was 10 times larger than the minimum inoculum that produces endocarditis in 90% of untreated rats. In the therapeutic experiments, one of the investigators examined the rats twice a day. After inoculation, two rats were killed by CO2 inhalation before the start of therapy because they presented signs of suffering, including curled-up position, ruffled fur, and loss of spontaneous activity. Data for these rats were not included in the data analysis. No rats were killed during therapy.
Antibiotic treatment of experimental endocarditis.
Antibiotic treatment was started 12 h after bacterial inoculation and continued for 3 days. The prodrug of BAL9141, BAL5788, was administered. Since no data from a phase I study with humans were available, we conducted a pilot study by infusing the drug continuously to obtain steady-state target levels in rat serum that were 5, 2.5, and 1.25 times higher than the MIC at which BAL9141 inhibited 90% of MRSA clinical isolates (12). This was achieved by concentrations in serum of 20, 10, and 5 mg/liter and required 288, 144, and 72 mg of BAL5788 per kg of body weight every 24 h, respectively. Amoxicillin-clavulanate (5:1 [wt/wt] ratio) and vancomycin were administrated at changing flow rates with the pump described above in order to simulate the kinetics of the drugs in the serum of humans during treatment with the antibiotics at 1.2 g i.v. every 6 h and 1 g i.v. every 12 h, respectively. This required 164.4 mg of amoxicillin-clavulanate (5:1 [wt/wt] ratio) per kg of body weight every 6 h (beginning with a bolus of 80 mg/kg, followed by a progressively decreasing infusion of the remaining 84.4 mg/kg) and 23.2 mg of vancomycin per kg of body weight every 12 h, respectively.
Pharmacokinetic studies.
The concentrations of antibiotics in serum were determined on day 2 of therapy with groups of three to five uninfected or infected rats per experiment. Drug levels in infected animals were determined from internal controls of the therapeutic experiments, in which adequate drug delivery was routinely monitored. Blood was drawn by puncturing the periorbital sinus of each rat (one puncture per animal) at different time points during and after antibiotic administration. Drug levels in the serum were measured by an agar diffusion bioassay with Bacillus subtilis ATCC 6633 as the indicator organism for BAL9141, amoxicillin, and vancomycin and with Klebsiella pneumoniae ATCC 29665 plus 60 mg of penicillin/liter for clavulanate (6). The diluent was pooled rat serum. The limits of detection of the assays were 0.07 mg/liter for BAL9141, 0.75 mg/liter for amoxicillin, 0.15 mg/liter for clavulanate, and 0.6 mg/liter for vancomycin. The linearities of the standard curves were assessed with a regression coefficient of ≥0.995, and intraplate and interplate variations were ≤10%.
Evaluation of infection.
Control rats were killed at the time of treatment onset, i.e., 12 h after the inoculation, in order to measure both the frequency and the severity of valve infection at the start of therapy. Rats treated with BAL9141 were killed 4 h after the end of administration of the last dose. Rats treated with amoxicillin-clavulanate or vancomycin were killed 8 h after the trough level of the last dose of antibiotic was attained in serum. At that time, no residual antibiotic concentration was experimentally detected in the serum except in animals treated with BAL9141. In the latter animals, the residual serum BAL9141 concentrations were less than 1 mg/liter (see Table 2). However, these low residual drug concentrations were unlikely to interfere with the valve cultures because (i) the residual concentrations of BAL9141 in rat serum were already lower than the MICs of the drug for the test organisms and (ii) processing of the valves before plating resulted in a dilution of the original sample ≥100 times.
TABLE 2.
Pharmacokinetic parameters for BAL9141 in the serum of ratsa
| BAL9141 target level (mg/liter) in serum | Actual levels (mg/liter) of BAL9141 in rat serum at the following times:
|
||||
|---|---|---|---|---|---|
| after start of infusion
|
4 h after end of infusion | ||||
| 18 h | 24 | 48 h | 72h | ||
| 5 | 6.1 ± 2.2 | 6.8 ± 2.6 | 6.2 ± 1.9 | 5.5 ± 2.6b | 0.21 ± 0.14 |
| 10 | 13.1 ± 1.2 | 12.8 ± 4.3 | 11.9 ± 4.2 | 14.5 ± 0.6b | 0.30 ± 0.13 |
| 20 | 29.2 ± 7.1 | 24.2 ± 8.5 | 25.5 ± 8.1 | 27.3 ± 5.2b | 0.89 ± 0.75 |
BAL5788, the prodrug of BAL9141, was administered as a continuous infusion, targeting steady-state BAL9141 levels in serum of 5, 10, and 20 mg/liter. Results are presented as the means ± standard deviations for three to nine determinations for individual animals.
P > 0.5 compared with values at 18 h.
The valve vegetations were dissected under sterile conditions, weighed, homogenized in 1 ml of saline, and serially diluted before being plated for determination of colony counts. Quantitative blood and spleen cultures were performed in parallel. Few animals died before the end of treatment due to either complications of the operation itself (such as possible catheter-induced arrhythmia) or the infection process, or both. Of these, only data for rats that had received at least 24 h of treatment were taken into account for vegetation bacterial counts. Blood and spleen cultures were not performed for these animals. The numbers of colonies growing on the plates were determined after 48 h of incubation at 35°C. The bacterial densities in the vegetations were expressed as log10 CFU per gram of tissue. The dilution technique permitted the detection of ≥2 log10 CFU/g of vegetation. For statistical comparisons of the differences between the median densities of bacterial vegetations in the various treatment groups, culture-negative vegetations were considered to contain 2 log10 CFU/g.
Detection of antibiotic resistance emergence in vivo.
To detect the emergence of antibiotic-resistant staphylococcal mutants during therapy for endocarditis, standard MICs were determined for bacteria which grew from vegetations on antibiotic-free agar. In this case, 1 in approximately 100 colonies growing from the infected vegetations was picked at random from the plates and grown in liquid culture, and the MIC of the drug to which the animal had been exposed was reassayed for the colony.
Statistical analysis.
Fisher’s exact test was used to compare the rates of valvular infection. Bonferroni’s correction was used for multiple-drug comparisons. Median bacterial densities in the vegetations were compared by the nonparametric Mann-Whitney Wilcoxon unpaired test. Overall, differences were considered significant at P levels of ≤0.05 by use of two-tailed significance levels.
RESULTS
Population analysis profile, antibiotic susceptibility, and time-kill curves.
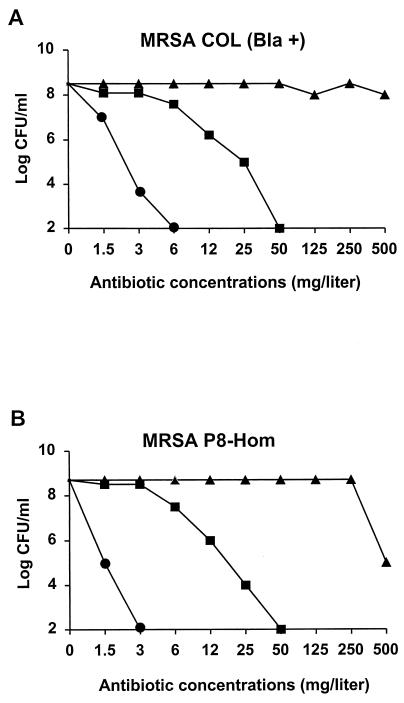
As shown by their population analysis profiles, both strain COL Bla+ and strain P8-Hom expressed homogeneous resistance to methicillin (Fig. 2). The population analysis profiles showed that both penicillinase-producing strains had homogeneous resistance to amoxicillin (data not shown), but amoxicillin had relatively good activity against the strains when clavulanate was added. In contrast, BAL9141 was active against both organisms, and neither organism grew on plates containing >3 mg of the drug per liter.
FIG. 2.
Population analysis profiles of homogeneously methicillin-resistant, penicillinase-producing strains COL Bla+ (A) and P8-Hom (B). Bacteria (>108 CFU) were spread onto agar plates containing increasing concentrations of methicillin (closed triangles), amoxicillin-clavulanate (closed squares), or BAL9141 (closed circles). Points indicate the numbers of colonies growing on the plates after 48 h of incubation at 35°C.
Table 1 shows the MICs of five antibiotics for the two MRSA strains used to infect the animals. As in the population analysis determinations, both organisms were highly resistant to methicillin. Amoxicillin alone was ineffective and required the addition of clavulanate to be relatively active against these penicillinase-producing organisms. BAL9141 was up to 10 times more active than either amoxicillin or amoxicillin-clavulanate. The MICs of BAL9141 were in the range of the previously reported MICs of BAL9141 for a panel of MRSA strains (12). Both strains were susceptible to vancomycin.
TABLE 1.
MICs of five antibiotics for the homogeneously methicillin-resistant, penicillinase-positive MRSA isolates used to induce experimental endocarditis
| Antibiotic | MIC (mg/liter)
|
|
|---|---|---|
| MRSA COL Bla+ | MRSA P8-Hom | |
| BAL9141 | 2 | 2 |
| Methicillin | >128 | >128 |
| Amoxicillin | >128 | >128 |
| Amoxicillin-clavulanate | 32 | 32 |
| Vancomycin | 2 | 2 |
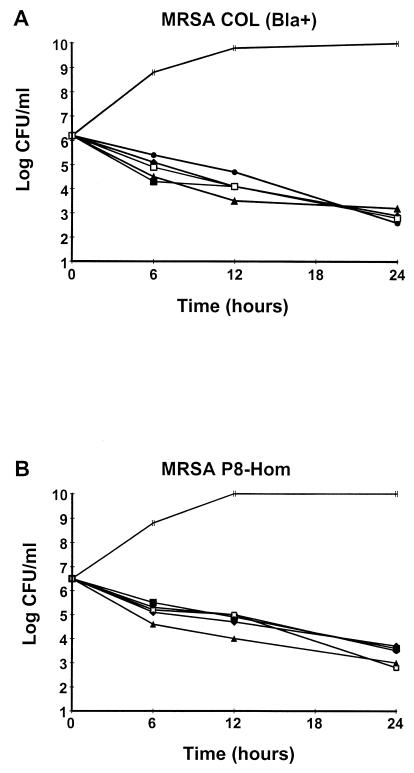
The killing experiments were done with antibiotic concentrations readily achieved in rat and/or human serum. BAL9141 showed typical time-dependent bactericidal activity (>2-log10 CFU/ml loss of viability within 24 h of exposure) at the three target concentrations (Fig. 3). The concentrations of amoxicillin-clavulanate (90 mg of amoxicillin per liter and 20 mg of clavulanate per liter) and vancomycin (40 mg/liter) chosen to simulate peak levels in serum inflicted a loss of viability on bacterial cultures similar to that achieved with BAL9141, whichever test organism was used.
FIG. 3.
Time-kill curves for the two penicillinase-producing MRSA strains used for the in vivo studies. The two strains were exposed to all three target steady-state concentrations of BAL9141 produced in the serum of rats (5, 10, and 20 mg/liter) or the peak concentrations of amoxicillin-clavulanate (90 mg of amoxicillin per liter and 20 mg of clavulanate per liter) or vancomycin (40 mg/liter) produced in humans and rats after the administration of therapeutic doses of the drugs. Each time-kill experiment was done on two separate occasions, with similar results on each occasion. Symbols: |, control; •, BAL9141 at 20 mg/liter; ⧫, BAL9141 at 10 mg/liter; ▴, BAL9141 at 5 mg/liter; ▪, vancomycin at 40 mg/liter; □, amoxicillin-calvulanate at 90 mg of amoxicillin per liter and 20 mg of clavulanate per liter.
Determination of PBP 2a affinity.
The binding affinities of BAL9141 and methicillin for PBP 2a were assessed by competition assay with [3H]penicillin. The IC50s of BAL9141 for inhibition of [3H]penicillin labeling of PBP 2a in membrane fractions of strain COL Bla+ and strain P8-Hom were 0.31 and 0.47 mg/liter, respectively. The IC50s of BAL9141 were >100 times lower than those of methicillin (39 and 78 mg/liter, respectively). This is in keeping with our earlier finding that the IC50s of amoxicillin were 40 times lower than those of methicillin (10, 18). Thus, the good in vitro activity of BAL9141 against the MRSA strains tested correlated with the greater affinity of this compound for PBP 2a compared with that of methicillin.
Penicillinase stability of BAL9141 in vitro.
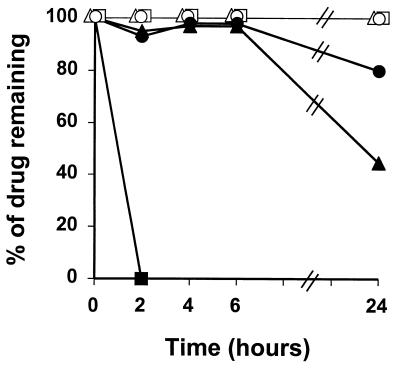
The stability of BAL9141 and the other antibiotics exposed to broth cultures containing large numbers (109 CFU) of either penicillinase-producing strain, COL Bla+ or P8-Hom, is shown in Fig. 4. Amoxicillin barely resisted degradation for a few minutes in this experiment (data not shown) (18), whereas in the presence of clavulanate, degradation took 2 h to go to completion. Methicillin was not completely exempt from penicillinase hydrolysis, loosing almost 20% of its original activity within 6 h of exposure and about 50% within 24 h. In contrast, BAL9141 was perfectly stable for 6 h and incurred a loss of only about 20% of its original activity within 24 h. Moreover, all three drugs were perfectly stable when exposed to broth cultures of the penicillinase-negative strain, COL Bla−.
FIG. 4.
In vitro degradation of BAL9141 (circles), amoxicillin-clavulanate (squares), and methicillin (triangles) exposed to large bacterial inocula (>109 CFU) of strain COL Bla+ (penicillinase producer; closed symbols) or COL Bla− (penicillinase negative; open symbols).
Antibiotic levels in rat serum.
BAL5788, the prodrug of BAL9141, was administered to rats by continuous infusion aimed at targeting steady-state BAL9141 levels in serum of 20, 10, and 5 mg/liter. The antibiotic levels obtained at different time points after the start of the infusion were some 20 to 40% (range, 19 to 57%) higher than planned (Table 2). Remarkably, these results show that the accumulation of BAL9141 did not occur with any of the regimens after 3 days of administration.
The simulations in rats of the kinetics of amoxicillin-clavulanate and vancomycin in human serum were as described previously (6). For instance, the levels of amoxicillin and clavulanate (means ± standard deviations for three determinations) measured in the serum of rats at 5 min (peak) and 6 h (trough) after the start of drug administration were 124.9 ± 13.0 and 0.86 ± 0.74 mg/liter, respectively, for amoxicillin, and 21.7 ± 1.72 and 0.41 ± 0.12 mg/liter, respectively, for clavulanate. The levels of vancomycin (means ± standard deviations for three determinations) measured in the serum of rats at 0.5 h (peak) and 12 h (trough) after the start of drug administration were 42.8 ± 0.25 and 4.85 ± 0.89 mg/liter, respectively.
Treatment of experimental endocarditis.
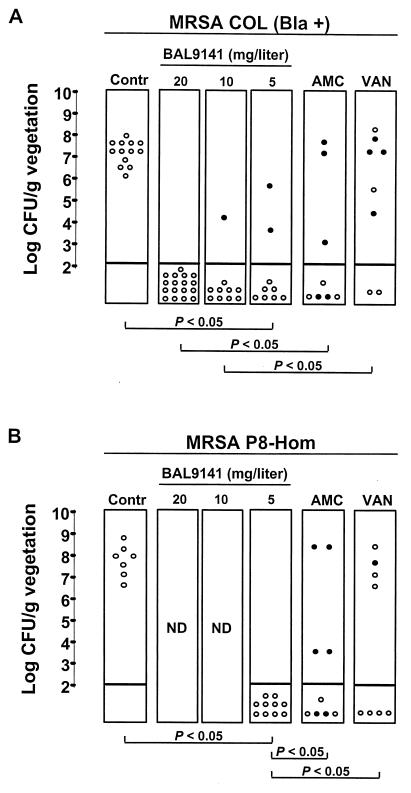
The results of a 3-day course of treatment of experimental endocarditis with BAL9141, amoxicillin-clavulanate, or vancomycin are presented in Fig. 5. Three regimens of BAL9141 given as continuous infusions targeted at achieving steady-state levels in serum of 20, 10, and 5 mg/liter were tested. All three BAL9141 regimens were successful in the treatment of experimental endocarditis due to the homogeneously methicillin-resistant and penicillinase-positive strain, strain COL Bla+ (Fig. 5A). Moreover, the highest steady-state level of BAL9141 in serum rendered more vegetations culture negative than therapy with amoxicillin-clavulanate or vancomycin (P < 0.05). Due to the good efficacies of the three BAL9141 regimens in vegetations infected with strain COL Bla+, only the lowest steady-state concentration (5 mg/liter) was tested in a second set of experiments against homogeneously methicillin-resistant and penicillinase-positive strain P8-Hom. This regimen produced 100% culture-negative vegetations (P < 0.05 compared with the results of amoxicillin-clavulanate or vancomycin treatment) (Fig. 5B).
FIG. 5.
Outcome of experimental endocarditis caused by homogeneously methicillin-resistant, penicillinase-producing S. aureus strains COL Bla+ (A) and P8-Hom (B) after 3 days of therapy. Each dot represents the bacterial density in the vegetation of a single animal. The closed dots represent the data for animals that died before the end of treatment. Panel A presents the results of two experiments pooled together. In one of them, the BAL9141 regimen with the highest concentration (20 mg/liter) was compared both to the control treatment and to the amoxicillin-clavulanate and vancomycin treatments. In the second experiment, the BAL9141 regimen with the highest concentration was compared to the control regimen and to the two lower-dose regimens. This explains the larger number of animals used as controls and the 20-mg/liter steady-state concentration in serum. P was <0.05 when the results were compared by Fisher’s exact test. Contr, control; AMC, amoxicillin-clavulanate; VAN, vancomycin; ND, not done.
Amoxicillin-clavulanate treatment decreased the bacterial densities in the vegetations by more than 4 log10 CFU/g compared to those in the vegetations of control rats (P < 0.05) infected with either MRSA strain. Compared to the untreated controls, vancomycin treatment achieved a reduction of <1 log10 CFU/g of vegetation for strain COL Bla+. It is noteworthy that four of eight (50%) of these animals died within 48 h of treatment. On the other hand, against strain P8-Hom vancomycin treatment produced a reduction of approximately 3 log10 CFU/g of vegetation compared to the counts in the vegetations of control rats, although this difference was not statistically significant.
Cultures of spleen and blood specimens from all available animals were negative. No resistant mutants were isolated from infected vegetations from any group of treated animals.
Thus, BAL9141 administered by continuous infusion was active against experimental endocarditis due to homogeneously methicillin-resistant and penicillinase-positive MRSA and was as effective or more effective than treatment with amoxicillin-clavulanate or vancomycin with regimens that simulate the kinetics in humans. The greater activity of BAL9141 was not due to differences in the times of killing after the end of therapy for at least two reasons. First, in a control experiment with the lower-dose regimen (5 mg/liter) used to achieve steady-state levels in serum and with killing at 8 h after the end of treatment, all animals had culture-negative vegetations (data not shown). Second, infected rats in the comparator groups had titers in vegetations several orders of magnitude greater than those in the vegetations from rats in the groups treated with BAL9141. The 4-h difference in posttherapy growth could not account for this difference when one considers the slow (ca. 1 h) doubling time of S. aureus in the vegetations (7).
DISCUSSION
The experiments presented herein demonstrate the efficacy of BAL9141, a novel intravenous cephalosporin, in the rat model of experimental endocarditis caused by homogeneously methicillin-resistant resistant, penicillinase-producing MRSA isolates. Indeed, all three BAL9141 regimens that achieved steady-state levels in serum of 2.5, 5, and 10 times the MIC for the test organisms sterilized almost all the vegetations in rats with experimental endocarditis caused by homogeneously methicillin-resistant, penicillinase-producing MRSA. Moreover, BAL9141 tended to render more vegetations culture negative than therapy with amoxicillin-clavulanate by a regimen that simulated the kinetics in humans. These results correlated with the results obtained in vitro. In vitro, BAL9141 showed a high affinity for PBP 2a, with an IC50 of about 0.5 mg/liter. By comparison, the IC50 was 4 mg/liter for amoxicillin, a β-lactam known for its relatively good affinity for PBP 2a (10, 18). Moreover, BAL9141 was more stable than amoxicillin-clavulanate to hydrolysis by penicillinase, even in the presence of large amounts of bacteria. This likely resulted in lower MICs of BAL9141 for MRSA compared to those of amoxicillin-clavulanate.
It is well established that some of the older β-lactams, such as penicillin, ampicillin, and amoxicillin, with relatively good affinities for PBP 2a are effective in vitro and in animals with experimental endocarditis due to penicillinase-negative MRSA (2, 8, 10, 15). In contrast, in animals with endocarditis caused by penicillinase-producing MRSA, they must be combined with a penicillinase inhibitor, such as sulbactam or clavulanate, in order to be effective (8, 10, 15). Moreover, a patient infected with a vancomycin-intermediate MRSA strain was successfully treated with ampicillin-sulbactam in Japan (14).
However, studies with animals have also demonstrated that there is still an unsettled issue in terms of bacterial penicillinase production. Experiments with rabbits have shown that the combination ampicillin-sulbactam or penicillin-sulbactam tends to be less effective against experimental infections due to penicillinase-producing MRSA than against experimental infections due to penicillinase-nonproducing MRSA (2, 8), mainly due to the incomplete inhibition of penicillinase produced at the site of infection. Moreover, cefamandole, a supposedly penicillinase-stable β-lactam, which remains essentially unaffected by penicillinase production in the presence of low concentrations of MRSA, such as the inoculum used in a standard MIC test, proved ineffective against experimental endocarditis caused by penicillinase-producing strains. Cefamandole was degraded by the large amounts of enzyme produced by the bacterial clusters packed in infected vegetations (18).
Thus, the results of our in vivo experiments highlight two important beneficial effects of BAL9141. First, whereas penicillinase production represents a serious restriction to the possible use of existing β-lactams with affinities for PBP 2a against infections caused by penicillinase-producing MRSA, the results available for BAL9141 suggest that use of this drug might solve this problem. Our findings agree with those in a recent publication describing a study with an experimental carbapenem (L-695,256) that demonstrated that the drug had in vivo activity in a rabbit model of MRSA endocarditis (1). Second, the good therapeutic results obtained with BAL9141 indirectly suggest that the diffusion of this drug into vegetations is likely to be adequate. The adequate penetration into tissue might account for the relatively superior activity of BAL9141 over that of therapy with vancomycin by a regimen that simulates the kinetics of the drug in humans, as vancomycin tends to poorly enter the core of the experimentally infected cardiac vegetations (5).
Since the precise pharmacokinetics of BAL9141 in humans have not yet been determined, the newly available i.v. form of the drug was administered as a continuous infusion to target levels in serum that exceed the MIC for the infecting organisms for 100% of the dosing interval. In rats, BAL9141 has a level of protein binding of ca. 20% (Basilea Pharmaceutica, data on file). When the protein-bound fraction (i.e., 20%) was subtracted, the serum BAL9141 levels remained above the MIC for the infecting bacteria for the entire dosing interval, even for the regimen with the lowest dose (5 mg/liter) that achieved steady-state levels. Although continuous infusion did not enable us to predict the optimal BAL9141 dosing regimens for application to clinical practice and might not be optimal for accurate comparisons with other drugs, it represents a valuable and reasonable approach to working out the antimicrobial effect of this drug in humans. Several experimental models have shown that the therapeutic efficacies of β-lactam antibiotics correlate with the duration of time that the levels in serum exceed the MIC for the infecting organisms, with maximal bacteriological efficacy predictably seen when the levels in serum remain above the MIC for about 30 to 50% of the dosing interval (3, 4). With regard to BAL9141, Andes and Craig showed that the level in serum that remains above the MIC for about 30% of the dosing interval is the parameter that best predicts the efficacy of a drug against MRSA in the neutropenic murine thigh infection model (D. R. Andes and W. A. Craig, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1079, p. 198, 2000).
In the present study, BAL9141 was active against MRSA in experimental endocarditis when it was used at steady-state levels in serum as low as two times the MIC for the infecting organisms. Due to both its low level of protein binding and the low MICs at which 90% of MRSA isolates are inhibited (12), it is reasonable to assume that these goals for concentrations above the MIC can be achieved with therapeutic doses in human serum.
Assessment of the efficacies of new antimicrobial agents against MRSA has become an urgent priority because MRSA isolates with reduced susceptibility to vancomycin and other glycopeptides have recently been described from geographically diverse sources. The experiments presented here demonstrated the efficacy of the novel cephalosporin BAL9141 in the treatment of experimental endocarditis in rats due to penicillinase-producing MRSA. The efficacy of BAL9141 in this difficult-to-treat infection further implies that β-lactams with both good affinities for PBP 2a and stabilities against penicillinase in vivo are worth future clinical investigations as potentially important alternatives for the treatment of MRSA infections.
Acknowledgments
We thank Malcom Page for help with PBP 2a binding affinity determinations and Marlyse Giddey and Jacques Vouillamoz for outstanding technical assistance.
REFERENCES
- 1.Chambers, H. F. 1995. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for the penicillin-binding protein PBP 2a. Antimicrob. Agents Chemother. 39:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, H. F., M. Kartalija, and M. Sande. 1995. Ampicillin, sulbactam, and rifampin combination treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J. Infect. Dis. 171:897–902. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Cremieux, A. C., B. Maziere, J. M. Vallois, M. Ottaviani, A. Azancot, H. Raffoul, A. Bouvet, J. J. Pocidalo, and C. Carbon. 1989. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159:938–944. [DOI] [PubMed] [Google Scholar]
- 6.Entenza, J. M., U. Fluckiger, M. P. Glauser, and P. Moreillon. 1994. Antibiotic treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J. Infect. Dis. 170:100–109. [DOI] [PubMed] [Google Scholar]
- 7.Entenza, J. M., Y. A. Que, J. Vouillamoz, M. P. Glauser, and P. Moreillon. 2001. Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance. Antimicrob. Agents Chemother. 45:3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantin, B., J. Pierre, N. Castela-Papin, L. Saint-Julien, H. Drugeon, R. Farinotti, and C. Carbon. 1996. Importance of penicillinase production for activity of penicillin alone or in combination with sulbactam in experimental endocarditis due tomethicillin-resistant Staphylococcus aureus. Antimicrob.Agents Chemother. 40:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fluckiger, U., P. Moreillon, J. Blaser, M. Bickle, M. P. Glauser, and P. Francioli. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franciolli, M., J. Bille, M. P. Glauser, and P. Moreillon. 1991. Beta-lactam resistance mechanisms of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 163:514–523. [DOI] [PubMed] [Google Scholar]
- 11.Goldman, P. L., and R. G. Petersdorf. 1980. Importance of beta-lactamase inactivation in treatment of experimental endocarditis caused by Staphylococcus aureus. J. Infect. Dis. 141:331–337. [DOI] [PubMed] [Google Scholar]
- 12.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. P. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63–9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heraief, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135–136. [DOI] [PubMed] [Google Scholar]
- 15.Hirano, L., and A. S. Bayer. 1991. β-lactam–β -lactamase-inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob. Agents Chemother. 35:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson, J. E. 2000. New gram-positive agents in nosocomial infection. Curr. Opin. Infect. Dis. 13:593–598. [DOI] [PubMed] [Google Scholar]
- 17.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212. [DOI] [PubMed] [Google Scholar]
- 18.Que, Y. A., J. M. Entenza, P. Francioli, and P. Moreillon. 1998. The impact of penicillinase on cefamandole treatment and prophylaxis of experimental endocarditis due tomethicillin-resistant Staphylococcus aureus. J. Infect.Dis. 177:146–154. [DOI] [PubMed] [Google Scholar]
- 19.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517–523. [DOI] [PubMed] [Google Scholar]
- 20.Tomasz, A., S. Nachmann, and H. Leaf. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]