Abstract
A “plasmid-curing effect” of multiresistant Escherichia coli by flavophospholipol, an antibiotic used as an antimicrobial growth promoter (AMGP) in animal feeds, has been reported to occur in vitro and in vivo under experimental conditions. In this study, the effect of flavophospholipol under field conditions was studied. The prevalence and degree (proportion of resistant strains to the total numbers present per gram of feces) of resistance of indicator bacteria, E. coli and enterococci, was determined in fecal samples from three groups of pigs that were fed a commercial finisher feed without any AMGP. Group A was the negative control group without any AMGP, group B received the same feed with 9 mg of flavophospholipol/kg of feed (study group), and group C received the same feed with 15 mg of avoparcin/kg (positive control). Fecal samples from each pig were collected at the start and at the end of the study and assessed for the prevalence and degree of resistance against antibiotics commonly used either for therapy in pig medicine or as an AMGP. Before the start of the study, all pigs were colonized with multiresistant E. coli by mixing three resistant pig isolates through their feed after disturbance of the colonization resistance of the intestinal flora by a 3-day course of lincomycin and spectinomycin. At the end of the study, the overall prevalence and degree of resistance of E. coli in the fecal flora had increased significantly in groups A and C but remained at the same level as at the start of the study in group B. The prevalence of vancomycin resistance was 44 and 41% in groups A and B, respectively, but only very low numbers of vancomycin-resistant enterococci (VRE) per gram of feces were found. In the avoparcin-fed group, the prevalence was 72%, and in 57% of the samples, more than 50% of all enterococci present were vancomycin resistant. The prevalence of resistant Enterococcus faecalis increased only in the flavophospholipol-exposed group, from 23% before the start of the study to 43% at the end of the study. It was concluded that flavophospholipol effectively suppressed the augmentation and dissemination of multiresistant E. coli in the intestinal flora of fattening pigs. Avoparcin use strongly selected for VRE carriage and excretion. Therefore, as neither flavophospholipol nor any related molecule is used therapeutically, no cross-resistance with therapeutic antibiotics exists and no transmissible resistance has been shown; the major decrease in resistance in intestinal E. coli of flavophospholipol-fed animals seemed to outweigh the small increase in the risk of transfer of flavophospholipol-resistant E. faecalis from animals to humans via the food chain.
The most effective way to decrease the prevalence and degree of resistance in fecal indicator bacteria is to reduce the amount of antibiotics used in a population. In food animals, antibiotics are not only used for therapy and prevention of bacterial infections but are also added continuously to animal feeds as antimicrobial growth promoters (AMGP) to enhance growth, improve feed conversion, and decrease waste production. In Europe, more than 30% of all antibiotics used in animals are used for growth promotion, but in some countries this percentage is even higher, e.g., in The Netherlands it is nearly 50% (56). This continuous feeding of antibiotics to animals causes a strong selection force for resistance against these and related antibiotics in particular (cross-resistance) and, via coselection, for multiresistance against other antibiotics the resistance genes for which are located on the same plasmid. This is the reason that several groups have proposed phasing out the use of antibiotics as AMGP (10, 21, 29, 42, 47).
The most important route of emergence and dissemination of resistance in bacteria in the gut is the spread of plasmids carrying resistance genes among the many species of bacteria in the intestinal flora of animals. Thus, if there were a safe substance that could be fed to animals to either eliminate resistance plasmids from intestinal bacteria or prevent the transfer of these plasmids among the bacteria, the prevalence and degree of resistance of bacteria in the intestinal flora of animals might be reduced significantly.
A “plasmid-curing” effect in Escherichia coli and other members of the family Enterobacteriaceae has been described for mitomycin, rifampin, and flavophospholipol and for nonantibiotic substances such as acridine orange, acriflavine, and nitroacridine II (7, 12). Of these molecules, only flavophospholipol has been licensed for use in pigs, calves, and poultry. Further research, however, has shown that flavophospholipol, as described for another phospholipid antibiotic, macarbomycin (36), had no plasmid-curing effect but selectively inhibited the growth of bacteria harboring certain plasmids. Normally, the MIC of flavophospholipol for E. coli isolates is >64 mg/liter, but in these plasmid-bearing strains, MICs range from 0.125 to 5 mg/liter (36). Therefore, flavophospholipol inhibits the growth of bacteria harboring certain R plasmids, but it has no effect on other strains (22, 46; T. Watanabe, Y. Ogata, K. Sugawara, and K. Oda, Proc. 7th Int. Congr. Chemother., abstr. A-8/11, 1971). Moreover, in vitro, flavophospholipol decreases the transfer frequency of some R plasmids in E. coli but has no effect on the transfer of other types of R plasmids (34, 48). These differences are most likely caused by an increased susceptibility to flavophospholipol of enterobacteria with sex pili and pilin protein precursors in the bacterial cell wall, and it has been speculated that flavophospholipol can enter the gram-negative bacterial cells via these structures (Watanabe et al., Proc. 7th Int. Congr. Chemother.).
Corpet, using a mouse model, showed that animals given flavophospholipol in their drinking water had significantly fewer tetracycline-resistant E. coli cells in their feces than control animals (11). Also, in studies with limited numbers of pigs and calves, addition of flavophospholipol to the feed as an AMGP caused a decrease in resistance against ampicillin, tetracyclines, streptomycin, and/or sulfonamides in fecal E. coli and/or salmonellae (13–15, 45, 49, 50). Rifampin has also been shown to decrease the prevalence of multiresistant E. coli in the fecal flora of pigs (30).
Flavophospholipol, also known as bambermycin or moenomycin, is a phosphorus-containing glycolipid antibiotic in which the lipid moiety (moenocinol) is a C-25 compound structurally analogous to undecaprenylphosphate (43) and is produced by a group of Streptomyces spp. including Streptomyces ghanaensis (12, 27, 52, 62). It inhibits peptidoglycan synthesis in the cell wall by inhibiting peptidoglycan polymerases through impairment of the transglycolase activities of penicillin binding proteins (PBPs) (26, 59, 60). There is, however, no cross-resistance between β-lactam antibiotics and flavophospholipol, as they act on different PBPs (41).
Flavophospholipol is mainly effective against gram-positive bacteria, i.e., staphylococci and streptococci, because it cannot penetrate the outer membrane of gram-negative bacteria. However, some activity against Pasteurella spp. and Brucella spp. has been reported (26). The spectrum of flavophospholipol is similar to that of penicillin and to some extent to that of macrolides. Enterobacteria, such as salmonellae and E. coli, are only slightly susceptible (34).
As flavophospholipol is not used therapeutically, no official breakpoints for resistance have been determined, and very few data on acquired resistance against flavophospholipol are available. Clostridium perfringens and Enterococcus faecium are considered intrinsically resistant (16, 19, 20), but susceptible strains of E. faecium have been reported recently (2). Differences among the PBPs in different species of enterococci might explain the different susceptibilities among enterococcal species (63). In a large collection of Staphylococcus aureus isolates from chickens and chicken slaughterers, no resistance against flavophospholipol was detected (25).
No publications about genes conferring resistance to flavophospholipol, transfer of resistance, or other resistance determinants in bacterial hosts were found, and no cross-resistance with other nonphosphorolipid class antibiotics has been described.
At the moment, flavophospholipol is used as an AMGP in pig, calf, and poultry feeds only. Neither flavophospholipol nor any related molecules are used for therapy in human or veterinary medicine.
Limited information is available on the effect of flavophospholipol-containing feeds on the intestinal flora of food animals, and the results of studies vary. In studies with pigs and calves, the duration of excretion and the numbers of salmonellae excreted were reduced (13–15), but in other studies using chickens, a decrease (5), no effect (23, 28), or a slight increase in excretion (44) was observed. Flavophospholipol has been shown to reduce the numbers of C. perfringens cells in the fecal flora of chickens despite the organism’s lack of susceptibility in vitro (5, 51).
Aim of the study.
This study was performed to examine the effect under field conditions of the use of flavophosholipol (Flavomycin; Intervet International, Wiesbaden, Germany) as an AMGP (9 mg/kg) in pig feed on the prevalence and degree of antibiotic resistance of E. coli and enterococci in the fecal flora of fattening pigs.
MATERIALS AND METHODS
Animals.
Three groups of fattening pigs, originating from the same breeder, were individually weighed at arrival and, based on weight and gender, equally divided into three groups of 56 animals each. Each group was housed in a separate compartment of the same pig stable under identical conditions. The animals had free access to feed, and water was supplied twice daily. Once daily, the animals were observed by an animal caretaker for general health status and for any clinical symptoms of disease, to insure a constant feed and water supply, and to check the proper functioning of heating and ventilation systems. The amounts fed to the pigs were recorded separately for each compartment. During the loading of the truck for transport to the abattoir, all pigs were individually weighed again.
During the first 4 weeks after arrival at the farm, all pigs were fed a starter pig diet containing 100 mg of tylosin (Tylan-100 premix 10%; Elanco Animal Health, Nieuwegein, The Netherlands)/kg, and during the study period, i.e., from week 5 until slaughtering at week 17, they were fed the same commercial finisher pig feed with the exception of differences in inclusions of AMGP. The pigs in compartment A (negative control) received feed containing no AMPG, those in compartment B (study group) received feed containing 9 mg of flavophospholipol/kg, and those in compartment C (positive control) received feed with avoparcin (15 mg/kg). During the study period, each person entering a compartment had to wash his or her hands and change into overalls and boots that were assigned to that compartment (different colors).
Colonization of the animals with multiresistant E. coli.
Immediately after arrival, all animals were treated orally with a lincomycin-spectomycin combination (Lincospectin 12.5%; Pharmacia & Upjohn Animal Health, Woerden, The Netherlands) for 3 days to disturb the intestinal flora and facilitate colonization by multiresistant E. coli strains. Lincospectin 12.5% (240 g) was dissolved in 2,400 ml of water. One hundred milliliters of this solution was thoroughly mixed through 7 kg of pig feed, and the feeding trough of each pen was filled with this mixture on days 1, 2, and 3. Overnight cultures of three multiresistant nonpathogenic E. coli strains, originally isolated from pig feces in a former study (39), in 800 ml of brain heart infusion (CM225; Oxoid, Basingstoke, United Kingdom) were mixed together, resulting in an E. coli mixture containing approximately 1.5 × 109 CFU of each strain per ml. Of this mixture, 13 ml was mixed through 1 kg of pig feed. After the troughs were emptied, if necessary, on the morning of day 3, 7 kg of feed, mixed with the bacterial suspension containing 4 × 1011 CFU of E. coli and the antibiotic solution, was provided to each pen containing seven animals. On days 4 and 5 again a freshly prepared culture identical to that used on day 3 but without the antibiotic solution was mixed through the feed, and again 7 kg was supplied to each pen.
Feces sampling.
During week 4, individual fecal samples of approximately 25 g from all pigs present in the three compartments were collected directly from the anus in plastic containers marked with the identification code of the pig and stored in a cool box with ice bags for transport to the laboratory. On the day of collection, the fecal samples were diluted 1:10 in the laboratory in peptone water containing 20% (vol/vol) glycerol, kept overnight at 4°C, and then frozen at −70°C and subsequently stored at −20°C until they were assayed. The same procedure was repeated in week 10 of the study and just before the pigs were slaughtered.
Bacteriological analysis.
After being thawed, the samples were inoculated on selective media with and without antibiotics. For E. coli, 37 μl of 10−1, 10−3, and 10−5 dilutions in 0.9% sodium chloride solutions were inoculated on eosin methylene blue agar plates (CM 69; Oxoid) using a spiral plater (Salm en Kip, Utrecht, The Netherlands); for enterococci, KF-Streptococcus agar plates (CM701; Oxoid) with the 10−1, 10−2, and 10−3 dilutions were used. For trimethoprim testing, 5% lysed horse blood was added to the agar. The antibiotics tested and the concentrations used are shown in Tables 1 and 2. These antibiotics were selected because of common use in human and veterinary medicine, as AMGP, or as having known cross-resistance with AMGP.
TABLE 1.
Prevalence and mean degree of resistance of strains of E. coli at the start of the study and at the end of the study in the separate compartmentsa
| Antibiotic | Concn in agar (mg/liter) | Start of study (n = 165)b
|
End of study
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Compartment A (n = 55)
|
Compartment B (n = 54)
|
Compartment C (n = 53)
|
|||||||
| Prev | MD | Prev | MD | Prev | MD | Prev | MD | ||
| Ampicillin | 50 | 99 | 15 | 100 | 82*# | 100 | 18 | 100 | 73*# |
| Streptomycin | 25 | 99 | 33 | 100 | 64*# | 100 | 36 | 100 | 62*# |
| Gentamicin | 25 | 21 | <1 | 78 | 2 | 13 | <1 | 17 | <1 |
| Oxytetracycline | 25 | 99 | 45 | 100 | 89*# | 100 | 31* | 100 | 78*# |
| Chloramphenicol | 25 | 66 | 4 | 100 | 6 | 96 | 3 | 98 | 10*# |
| Trimethoprim | 8 | 99 | 19 | 100 | 49*# | 98 | 27 | 100 | 43*# |
| Sulfamethoxazole | 150 | 99 | 37 | 100 | 71*# | 100 | 43 | 100 | 59* |
| Ampicillin-oxytetracycline | 50/25 | 99 | 8 | 100 | 68*# | 100 | 10 | 100 | 65*# |
n, number of samples; Prev, prevalence; MD, mean degree of resistance; <1, below detection level. Statistical significance: *, P < 0.05 in comparison to the start of the study; #, P < 0.05 compartment A in comparison with compartment B; #, P < 0.05 compartment C in comparison with compartment B.
Total of compartments A, B, and C.
TABLE 2.
Prevalence and mean degree of resistance of fecal enterococci at the start of the study and at the end of the study in the separate compartmentsa
| Antibiotic | Concn in agar (mg/liter) | Start of study (n = 165)b
|
End of study
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Compartment A (n = 55) | Compartment B (n = 54) | Compartment C (n = 53) | |||||||
| Prev | MD | Prev | MD | Prev | MD | Prev | MD | ||
| Gentamicin | 1,000 | 63 | 9 | 7 | <1 | 26 | 1 | 25 | <1 |
| Ampicillin | 25 | 0 | <1 | 4 | <1 | 0 | <1 | 0 | <1 |
| Erythromycin | 8 | 99 | 81 | 100 | 81 | 100 | 86 | 100 | 93* |
| Flavophospholipol | 8 | 23c | 22 | 13c | 12 | 43c | 11 | 28c | 2* |
| Vancomycin | 8 | 0 | <1 | 44 | 1 | 41 | 5 | 72 | 89*# |
n, number of samples; Prev, prevalence; MD, mean degree of resistance; <1, below detection level. Statistical significance: *, P < 0.05 in comparison to the start of the study; #, P < 0.05 compartment A in comparison with compartment B; #, P < 0.05 compartment C in comparison with compartment B.
Total of compartments A, B, and C.
Only E. faecalis.
E. coli grows on eosin methylene blue agar as purple colonies with black centers and a metallic shine. Only these colonies were counted after 18 to 24 h of incubation at 37°C. The minimum detection level was 300 CFU per g of feces. It has been shown that more than 95% of the colonies presumptively identified in this way are E. coli (41) and that the MICs for all isolates from antibiotic-containing plates were identical to or higher than the concentrations of the antibiotics in the selective plates from which they had been isolated (6, 35, 39).
Enterococci typically appear as red or pink colonies on KF-Streptococcus agar. After 48 h of incubation at 42°C, only the typical pink colonies were counted. One randomly sampled typical colony was collected from each KF-Streptococcus agar plate without antibiotics and identified using generally accepted methods (18). All isolates proved to belong to the genus Enterococcus. The minimum detection level was 300 CFU/g of feces. For the prevalence of resistance against flavophospholipol, a specimen was only considered positive if at least one Enterococcus faecalis cell was isolated and identified from the flavophospholipol-containing agar plate. For this reason, for flavophospholipol, only the prevalence of resistance could be calculated. In a previous study using the same methodology, the MICs for all enterococci were identical to or higher than the concentrations of the antibiotics in the selective plates from which they had been isolated (55).
Identification of and determination of MICs for enterococcal isolates.
From each antibiotic-free control plate, one colony was randomly picked and identified using API-strep (Api-Benelux, Den Bosch, The Netherlands) and the criteria of Devriese et al. (17, 18).
The MICs for the isolates were determined using the NCCLS agar incorporation method. E. faecalis (ATCC 51299) was used as the control strain.
Statistical analysis.
To estimate the differences in prevalence and percentages of a high degree of resistance among the groups, Pearson’s chi square test and, where appropriate, Fisher’s exact test were used. For the degree of resistance, a one-way analysis of variance was used to estimate overall differences between the group means. Group means were compared in pairs using the Student t test to control for overall error rate (Bonferroni test), and a P value of <0.05 was regarded as statistically significant.
Definitions.
The prevalence of resistance (percent) in the population for a certain antibiotic was calculated as the number of samples showing growth of E. coli or enterococci on the plates containing that antibiotic divided by the total number of samples tested times 100%.
The degree of resistance (percent) of each fecal sample to each of the antimicrobial agents tested was calculated as the number of CFU growing on the plate containing that agent divided by the total number of typical colonies on the antibiotic-free control plate times 100%.
The mean degree of resistance to an antibiotic was defined as the mean of the degrees of resistance against that antibiotic of the samples that were found to contain E. coli or enterococci resistant against that antibiotic.
RESULTS
During the study, six animals died (one in group A, two in group B, and three in group C), mostly from endocarditis. Apart from some respiratory problems in individual animals, a few abscesses, and a broken pelvis, there were no serious health problems observed during the study. The numbers of individual antibiotic treatments for either respiratory problems or severe wounds caused by fighting were four injections in group A, eight in group B, and eight in group C. The majority of these treatments were given during the first 3 weeks after the arrival of the pigs at the farm. Apart from the studied AMGP and six injections with a combination of procaine benzylpenicillin and dihydrostreptomycin sulfate (Streptoprocpen; AUV, Cuijk, The Netherlands), no antibiotics were given therapeutically or as prophylactics during the study period. There were no statistically significant differences in growth rate and feed conversion among the three compartments.
E. coli.
The mean ± standard error of the mean of the total numbers (log10 CFU per gram of feces) of E. coli cells in the fecal samples of all pigs collected at the start of the study was 6.5 ± 0.1. There were no significant differences among the three compartments at the start of the study. At the end of the study, in compartment B, the total number of E. coli cells (6.2 ± 0.1) was significantly lower than at the start, and in compartment A, the total number (6.9 ± 0.1) was higher (P < 0.05). For compartment C (6.4 ± 0.1), there was no significant difference. The total number of E. coli cells in compartment A at the end of the study was significantly higher than those in compartments B and C (P < 0.05).
The prevalence and mean degree of resistance of resistant E. coli in the fecal samples of the pigs collected at the start of the study and at the end of the study are shown in Table 1. No differences among the three compartments were observed at the start of the study (data not shown). There was no difference in prevalence of resistance at the start compared with the prevalences found at the end of the study in all three compartments for ampicillin, streptomycin, oxytetracycline, trimethoprim, sulfamethoxazole, and the combination of ampicillin and oxytetracycline. The prevalence of chloramphenicol resistance increased significantly (P < 0.001) during the study in all three compartments, but gentamicin resistance increased significantly only in compartment A (P < 0.001). At the end of the study, the prevalence of resistant E. coli in fecal samples from compartment B was significantly lower for gentamicin than in samples from compartments A and C (P < 0.001).
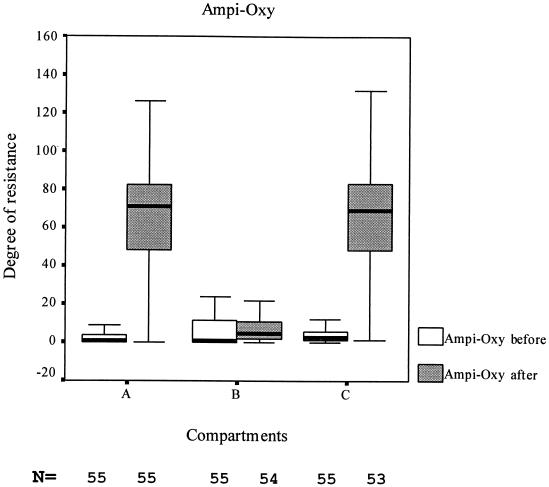
Similar differences were obtained in comparing the mean degree of resistance found before the start of the study and in the three different groups at the end of the study. In the compartments without flavophospholipol, A and C, the mean degree of resistance for all antibiotics tested was significantly (P < 0.05) higher at the end of the study than at the start of the study. Exceptions were, as shown in Table 1, the mean degree of gentamicin resistance, which did not change in any of the three groups during the study period, and the mean degree of chloramphenicol resistance, which increased only in group C. In contrast, in the flavophospholipol-exposed group, the mean degree of resistance to the tested antibiotics did not change during the study and even decreased significantly for oxytetracycline. Comparison of the mean degrees of resistance to the tested antibiotic of the different compartments at the end of the study showed that for nearly all antibiotics the mean degree of resistance was significantly lower (P < 0.05) in the flavophospholipol-exposed group B than in either of the other groups.
As a typical example, the differences in ampicillin- and oxytetracycline-resistant E. coli in fecal samples of all pigs at the start of the study and at the end in compartments A, B, and C is shown in Fig. 1 by means of a box plot.
FIG. 1.
Boxplot of degrees of resistance of E. coli before and after the study. Ampi-Oxy, combination of ampicillin and oxytetracycline.
Enterococci.
At the start of the study, there were no differences among the three compartments, and the mean ± standard error of the mean of the total numbers (log10 CFU per gram of feces) was 5.0 ± 0.1. At the end of the study, the total numbers of enterococci in all three compartments were significantly higher than at the start (P < 0.05). At the end of the study, the total numbers of enterococci in fecal samples from compartments A (5.5 ± 0.1) and B (5.4 ± 0.1) were significantly (P < 0.05) lower than in those from compartment C (6.0 ± 0.1), but no significant difference was found between compartments A and B.
The prevalence of resistant enterococci and the percentage of samples with a high proportion (>50%) of resistant enterococci in the fecal samples from pigs collected at the start and at the end of the study are shown in Table 2.
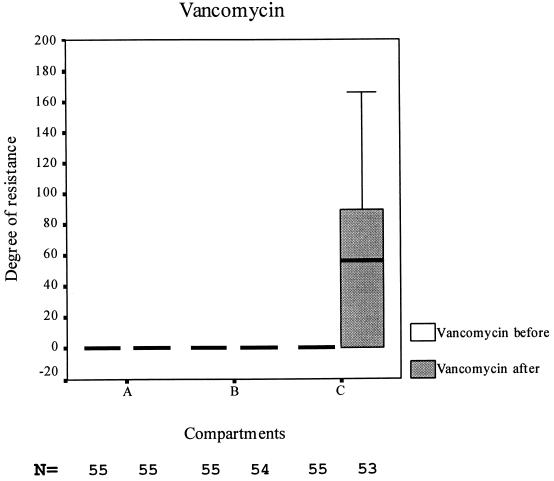
No differences among the three compartments were observed at the start of the study (data not shown). There was no significant difference in prevalence of resistance found in samples taken before the start and at the end of the study for ampicillin and erythromycin. In all the compartments, the prevalence of gentamicin resistance was lower at the end of the study than at the start (P < 0.001), but in compartment A it was also significantly lower than in the other two compartments (P < 0.01). The prevalence of vancomycin resistance increased significantly in all three compartments (P < 0.001). The prevalence in groups A and B increased to approximately 44%, but only very few vancomycin-resistant enterococci (VRE) were present in the positive fecal samples; i.e., the degree of vancomycin resistance remained low, mostly only 1 to 2%. In compartment C, however, the prevalence of VRE was not only significantly (P < 0.01) higher than in compartments A and B, but also, the mean degree of resistance was significantly higher and approximately 90% of the enterococci excreted by these animals were VRE (Table 2 and Fig. 2). The prevalence of flavophospholipol-resistant E. faecalis decreased during the study in compartment A and increased significantly (P < 0.05) in compartment B.
FIG. 2.
Boxplot of degrees of resistance of fecal enterococci before and after the study.
Identification and MIC distribution.
The identifications of the single isolates from fecal samples collected before and at the end of the study are shown in Table 3. There were no significant differences in species and MIC distribution among the three compartments at the start of the study (data not shown). A replacement of E. faecium during the study by E. faecalis, considered intrinsically flavophospholipol-resistant, was observed in the flavophospholipol-fed animals.
TABLE 3.
Identification of fecal enterococci from pigs before (A + B + C) and at the end of the study (compartments A, B, and C)
| Species | No. of samples
|
|||
|---|---|---|---|---|
| Before (n = 68)a | End
|
|||
| A (n = 55) | B (n = 54) | C (n = 53) | ||
| E. faecalis | 11 | 20 | 2 | 3 |
| E. faecium | 35 | 26 | 32 | 45 |
| E. durans | 122 | 6 | 20 | 7 |
| E. gallinarum | 1 | |||
| E. casseliflavus | 2 | |||
Total of A, B, and C.
All isolates were resistant to erythromycin (MIC > 64 mg/liter).
The MIC distributions of the E. faecium, E. faecalis, and Enterococcus durans isolates are shown in Table 4.
TABLE 4.
MIC distribution before (compartments A + B + C) and at the end of the study (compartments A, B, and C)
| Bacterium and antimicrobial | Compartment | % Resistant | No. of isolates with MIC (mg/liter) of:
|
MIC50 | MIC90 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.12 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | |||||
| E. faecium | ||||||||||||||||
| Flavophospholipol | A + B + C | 97 | 1 | 2 | 9 | 10 | 8 | 5 | 16 | 64 | ||||||
| A | 100 | 3 | 23 | 16 | 16 | |||||||||||
| B | 94 | 1 | 1 | 2 | 14 | 14 | 8 | 16 | ||||||||
| C | 95 | 1 | 1 | 39 | 1 | 1 | 8 | 8 | ||||||||
| Vancomycin | A + B + C | 0 | 29 | 3 | 3 | 0.5 | 1 | |||||||||
| A | 0 | 23 | 3 | 0.5 | 0.5 | |||||||||||
| B | 16 | 23 | 4 | 1 | 4 | 1 | >64 | |||||||||
| C | 86 | 5 | 1 | 37 | >64 | >64 | ||||||||||
| E. faecalis | ||||||||||||||||
| Flavophospholipol | A + B + C | 18 | 4 | 2 | 1 | 2 | 1 | 1 | 0.5 | 32 | ||||||
| A | 0 | 1 | 19 | 0.25 | 0.25 | |||||||||||
| B | 0 | 2 | 0.25 | 0.25 | ||||||||||||
| C | 0 | 2 | 1 | 0.25 | 0.5 | |||||||||||
| Vancomycin | A + B + C | 0 | 3 | 8 | 1 | 1 | ||||||||||
| A | 50 | 5 | 5 | 10 | 2 | 4 | ||||||||||
| B | 0 | 2 | 1 | 1 | ||||||||||||
| E. durans | ||||||||||||||||
| Flavophospholipol | A + B + C | 88 | 0 | 0 | 15 | 28 | 10 | 18 | 16 | 26 | 9 | 16 | 64 | |||
| A | 100 | 5 | 1 | 8 | 8 | |||||||||||
| B | 30 | 1 | 1 | 12 | 1 | 2 | 3 | 2 | >64 | |||||||
| C | 83 | 1 | 1 | 1 | 2 | 1 | 16 | 64 | ||||||||
| Vancomycin | A + B + C | 0 | 61 | 25 | 36 | 0.5 | 2 | |||||||||
| A | 0 | 1 | 4 | 1 | 1 | 1 | ||||||||||
| B | 0 | 2 | 18 | 1 | 1 | |||||||||||
| C | 17 | 2 | 3 | 1 | 1 | 1 | ||||||||||
DISCUSSION
After arrival at the farm but prior to the study, all pigs were vaccinated against commonly occurring pig diseases and given tylosin for 4 weeks to minimize the chance of an infectious disease outbreak during the study period that would have required antibiotic treatment of all pigs in the affected compartment(s), as this would have severely interfered with the study. To ascertain that multiresistant E. coli was present in all three study groups, three strains of multiresistant E. coli were mixed through the feed of all animals. To facilitate colonization by at least one of these strains, the colonization resistance (CR) of the intestinal tract had been disturbed by a short course of antibiotics with high activity against the obligate anaerobic flora (58, 61). Except for a few parenteral treatments of individual pigs, no antibiotics other than the studied AMGP were given to the pigs during the study period.
In a comparison of the three groups of pigs, the growth rate and feed intake tended to be less in the group of animals not receiving any AMGP than in the other two groups. This resulted in slightly better feed conversion. However, it was not statistically significant. The total numbers of E. coli cells and enterococci per gram of feces at the start of the study were of the same order as the results obtained in a former study (53). The total numbers of enterococci per gram of feces increased during the study in all three groups, but significantly more in the avoparcin-exposed group. In contrast, the total numbers of E. coli cells decreased in the flavophospholipol group, increased in the negative control group, and remained stable in the avoparcin group. According to the concept of CR, (part of) the endogenous obligate anaerobic flora limits the concentration of (potentially) pathogenic bacteria in the intestinal tract (57, 58). Impairment of the CR of the intestinal tract causes an increase in the fecal concentration of facultative aerobic enterobacteria as well as enterococci, if resistant (or nonsusceptible) strains are present (61). At the end of the study, the total numbers of both E. coli cells and enterococci per gram of feces were lower in the flavophospholipol group than in the avoparcin group. Within the flavophospholipol group, the total number of E. coli cells was lower at the end than at the start of the study. The total number of enterococci in the fecal flora of flavophospholipol-exposed pigs increased during the study, but less than in the other two groups of pigs. Therefore, it might be concluded that in flavophospholipol-treated animals the CR was not affected or was less affected than in either of the other groups of pigs. The increase in the numbers of E. coli cells in the negative control group A is difficult to explain but might have been caused by bacterial overgrowth in the small intestinal tract, which was controlled in the other groups by the AMGP. As the CR of the intestinal tract also protects against colonization with salmonellae, these observations are in concordance with the results of others. In flavophospholipol-fed pigs, a reduction in the number of salmonellae per gram of feces and in the duration of excretion of salmonellae has been observed (15). In avoparcin-fed animals, however, the minimal infectious dose of salmonellae is decreased and numbers and duration of excretion are increased compared to controls (3, 4, 24).
E. coli.
Resistance in fattening pigs increases with age, crowding, and stress, caused by, e.g., high temperatures and transport (8, 33, 37). Some of these factors might be interrelated, such as age, transport, and holding time at the abattoir. It has been shown that in pigs the proportion of resistant E. coli is higher in the fecal contents than in the colon. Stress enhances the transit time in the intestinal tracts of pigs and thus the numbers of resistant bacteria per gram of feces, because these numbers are higher in the cecum (37). In this study, there were neither differences in housing conditions nor other stress factors or relevant differences in therapeutic use of antibiotics among the three study groups. Therefore, the observed differences must be due to the differences in the AMGP supplied. The observed high prevalence of antibiotic-resistant E. coli is in accordance with previous studies (40, 53). Resistance against nearly all antibiotics with the exception of chloramphenicol and gentamicin was found in almost all pigs at the start and at the end of the study. It was interesting that in groups A and C the degree of resistance to ampicillin, streptomycin, oxytetracycline, trimethoprim, and the combination of oxytetracycline and ampicillin was significantly higher at the end of the study than at the start. In the flavophospholipol group, however, the degree of resistance remained on the same order for almost all antibiotics and was even lower for oxytetracycline. The animals in this group excreted considerably lower numbers of resistant E. coli cells in their feces, indicating that the reservoir of resistance genes present in the intestinal flora of these animals was much smaller than in the other two groups. Flavophospholipol in a concentration of 9 mg/kg added to the feed of fattening pigs prevented either overgrowth by resistant strains or transmission of plasmids carrying resistance genes among intestinal E. coli of these animals. Both phenomena have also been observed in vitro (7, 22, 34, 46) and in vivo in experiments with small numbers of animals kept under laboratory conditions (13,14,15). Consequently, the chance for transfer of resistance genes to other bacteria in the intestinal tracts of these animals, and the risk of transfer of resistant E. coli via meat (products) to humans, decreased as well.
Enterococci.
The observed high prevalence and degree of resistance of enterococci in all three groups against erythromycin were to be expected. Just before the start of the study, all of the animals were treated with tylosin, which is known to select for erythromycin resistance in fecal enterococci of pigs (1, 9).
At the start of the study, the number of VRE in all pigs was below the detection level of the method used. The increase in both prevalence and degree of resistance in the avoparcin-fed group was most striking. Of all excreted enterococci per gram of feces in VRE-positive animals, 71% were VRE. Prevalence of VRE among Dutch pigs used to be very general as a result of the common use of avoparcin as an AMGP. Since avoparcin has been banned as an AMGP, the prevalence and degree of resistance have been gradually declining (54). Therefore, it is most likely that at the start of the study a few animals carried VRE below the detection level. Because of exposure to avoparcin, these had been selected out and disseminated extensively in group C. The increase in the other groups is more difficult to explain, but an increase in the prevalence and degree of resistance of VRE in pig herds not exposed to avoparcin with increasing age of the animals has also been described by others (8). Because of the thorough separation of the compartments during construction and strict barrier procedures, cross-contamination was not considered a large risk, but it could not be absolutely excluded. However, if despite all preventive measures taken some carryover of resistant bacteria occurred during the study period, this could only have decreased the observed differences among the three groups of animals. Gentamicin resistance, however, was reduced in the avoparcin group during the study and was significantly lower at the end of the study than in either of the other groups. This might have been due to a replacement of gentamicin-resistant non-E. faecium enterococcal species by vancomycin-resistant E. faecium strains. Vancomycin resistance is more common in E. faecium, and gentamicin resistance is more common in E. faecalis, as indicated in Table 4. The prevalence of flavophospholipol-resistant E. faecalis increased in the flavophospholipol group during the study, but the numbers of flavophospholipol-resistant enterococci excreted per gram of feces (degree of resistance) even decreased.
The results of the MIC determinations of single isolates clearly indicated that mass medication with tylosin causes a selection and spread of erythromycin resistance in all enterococcal species. Avoparcin selects for vancomycin-resistant E. faecium. Like E. faecium, E. durans is probably nonsusceptible to flavophospholipol, which is not very surprising, as E. durans is closely related to E. faecium and has long been considered a subtype of that species (38). However, in this study, vancomycin resistance was not observed in E. durans. No resistance against flavophospholipol emerged during the study in the flavophospholipol-fed group B or in the avoparcin group, but nearly all E. faecalis cells were eradicated from the fecal flora of pigs in these two groups, as shown in Table 3.
Conclusions.
As was to be expected from the results reported in other publications (2, 32, 64), the use of avoparcin as an AMGP selected strongly for vancomycin-resistant enterococci in the fecal flora of exposed pigs. It was not surprising that in the flavophospholipol-fed group the prevalence of intrinsically flavophospholipol-resistant E. faecalis increased at the expense of E. faecium, a species which is generally susceptible. This selection was also observed in another study in which pigs were fed mupirocin as an AMGP (31). E. faecalis is also intrinsically resistant to mupirocin, and E. faecium is generally susceptible. However, despite this, the prevalence and mean degree of resistance of flavophospholipol-resistant enterococci also decreased during the study in the flavophospholipol group, but less than in either of the other groups.
Addition of flavophospholipol to the feed almost totally prevented the increase in numbers of resistant E. coli cells in the fecal flora of group B. As a result, the numbers of resistant E. coli cells excreted with the feces in the flavophospholipol group were significantly lower than in the other two groups. Consequently, use of flavophospholipol as an AMGP might decrease the risk of dissemination of resistant gram-negative bacteria and plasmid-mediated resistance genes from animals to humans via the food chain. In a risk-benefit analysis, the benefits of adding flavophospholipol to pig feed in order to reduce resistance might be considered greater than the potential risk of selection for flavophospholipol-resistant E. faecalis in the intestinal tracts of pigs and transfer to humans. However, as this study was performed on only one farm with pigs previously colonized with only three multiresistant E. coli strains, the results have to be confirmed in a field study comparing pigs from a number of different farms using flavophospholipol as an AMGP with others that do not. It would also be interesting to investigate whether the addition of flavophospholipol to pig feed prevents an increase in the prevalence and degree of antibiotic-resistant E. coli in the intestinal tracts of animals when they are treated with antibiotics, such as ampicillin, oxytetracycline, and trimethoprim-sulfonamide combinations.
REFERENCES
- 1.Aarestrup, F. M., and B. Carstensen. 1998. Effect of tylosin used as a growth promoter on the occurrence of macrolide-resistant Enterococci and Staphylococci in pigs Microb. Drug Resist. 4:307–312. [DOI] [PubMed] [Google Scholar]
- 2.Bager, F. 1999. Danmap 98. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Danish Zoonosis Centre, Copenhagen, Denmark.
- 3.Barrow, P. A. 1989. Further observations on the effect of feeding diets containing avoparcin on the excretion of salmonellas by experimentally infected chickens. Epidemiol. Infect. 102:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow, P. A., H. Williams Smith, and J. F. Tucker. 1984. The effect of feeding diets containing avoparcin on the excretion of salmonellas by chickens experimentally infected with natural sources of salmonella organisms. J. Hyg. 93:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolder, N. M., J. A. Wagenaar, F. F. Putirulan, K. T. Veldman, and M. Sommer. 1999. The effect of flavophospholipol (Flavomycin (R)) and salinomycin sodium (Sacox (R)) on the excretion of Clostridium perfringens, Salmonella enteritidis, and Campylobacter jejuni in broilers after experimental infection. Poultry Sci. 78:1681–1689. [DOI] [PubMed] [Google Scholar]
- 6.Bonten, M., E. E. Stobberingh, J. J. Philips, and A. Houben. 1990. High prevalence of antibiotic resistant Escherichia coli in faecal samples of students in the south-east of The Netherlands. J. Antimicrob. Chem. 26:585–592. [DOI] [PubMed] [Google Scholar]
- 7.Brana, H., J. Hubacek, and J. Konig. 1974. The effect of actinomycin D and flavomycin on Escherichia coli R+ strains. Folia Microbiol. 18:257–259. [DOI] [PubMed] [Google Scholar]
- 8.Butaye, P., L. A. Devriese, H. Goossens, M. Ieven, and F. Haesebrouck. 1999. Enterococci with acquired vancomycin resistance in pigs and chickens of different age groups. Antimicrob. Agents Chemother. 43:365–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie, P. J., J. N. Davidson, R. P. Novick, and G. M. Dunny. 1983. Effects of tylosin feeding on the antibiotic resistance of selected gram-positive bacteria in pigs. Am. J. Vet. Res. 44:126–128. [PubMed] [Google Scholar]
- 10.Committee on Antimicrobial Growth Promoters. 1998. Antimicrobial growth promoters, 15th ed. Health Council of The Netherlands, Rijswijk, The Netherlands.
- 11.Corpet, D. E. 1984. The effect of bambermycin, carbadox, chlortetracycline and olaquindox on antibiotic resistance in intestinal coliforms: a new animal model. Ann. Microbiol. 135:329–339. [DOI] [PubMed] [Google Scholar]
- 12.Crawford, L. M. 1984. Bambermycins, p. 351–354. In T. H. Jukes, H. L. Dupont, and L. M. Crawford (ed.), Antibiotics, sulphonamides, and public health, vol. 1. CRC Press, Boca Raton, Fla.
- 13.Dealy, J., and M. W. Moeller. 1977. Effect of bambermycins on Escherichia coli and antibiotic resistance in calves. J. Anim. Sci. 45:1239–1242. [DOI] [PubMed] [Google Scholar]
- 14.Dealy, J., and M. W. Moeller. 1977. Influence of bambermycins on Salmonella infection and antibiotic resistance in calves. J. Anim. Sci. 44:734–738. [DOI] [PubMed] [Google Scholar]
- 15.Dealy, J., and M. W. Moeller. 1976. Influence of bambermycins on Salmonella infection and antibiotic resistance in swine. J. Anim. Sci. 42:1331–1336. [DOI] [PubMed] [Google Scholar]
- 16.Devriese, L. A., G. Daube, J. Hommez, and F. Haesebrouck. 1993. In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth enhancing antibiotics. J. Appl. Microbiol. 75:55–57. [DOI] [PubMed] [Google Scholar]
- 17.Devriese, L. A., J. Hommez, R. Wijfels, and F. Haesebrouck. 1991. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Bacteriol. 71:46–50. [PubMed] [Google Scholar]
- 18.Devriese, L. A., A. V. D. Kerckhove, R. Klipper-Balz, and K. H. Schleifer. 1987. Characterization and identification of Enterococcus species isolated from the intestines of animals. Int. J. Syst. Bacteriol. 37:257–259. [Google Scholar]
- 19.Dutta, G. N., and L. A. Devriese. 1984. Observations on the in vitro sensitivity and resistance of Gram positive intestinal bacteria of farm animals to growth promoting antimicrobial agents. J. Appl. Bacteriol. 56:117–123. [DOI] [PubMed] [Google Scholar]
- 20.Dutta, G. N., and L. A. Devriese. 1980. Susceptibility of Clostridium perfringens of animal origin to fifteen antimicrobial agents. J. Vet. Pharmacol. Therapeut. 3:227–236. [Google Scholar]
- 21.European Commission. 1999. Opinion of the scientific steering committee on antimicrobial resistance. Directorate general XXIV: consumer policy and consumer health protection. European Commission, Brussels, Belgium.
- 22.George, B. A., and D. J. Fagerberg. 1984. Effect of bambermycins, in vitro, on plasmid-mediated antimicrobial resistance. Am. J. Vet. Res. 45:2336–2341. [PubMed] [Google Scholar]
- 23.George, B. A., D. J. Fagerberg, C. L. Quarles, J. M. Fenton, and G. A. McKinley. 1982. Effect of bambermycins on quantity, prevalence, duration, and antimicrobial resistance of Salmonella typhimurium in experimentally infected broiler chickens. Am. J. Vet. Res. 43:299–303. [PubMed] [Google Scholar]
- 24.Gustafson, R. H., J. R. Beck, and J. D. Kobland. 1982. The influence of avoparcin on the establishment of Salmonella in chickens. Zentbl. Veterinarmed. B 29:119–128. [DOI] [PubMed] [Google Scholar]
- 25.Hentschel, S., D. Kusch, and H. J. Sinell. 1979. Staphylococcus aureus in poultry, biochemical characteristics, antibiotc resistance and phage pattern. Zentbl. Bakteriol. Hyg. Abt. 1 Orig. B. 168:548–561. [PubMed] [Google Scholar]
- 26.Huber, G., and G. Nesemann. 1968. Moenomycin, an inhibitor of cell wall synthesis. Biochem. Biophys. Res. Commun. 30:7–13. [DOI] [PubMed] [Google Scholar]
- 27.Huber, G., U. Schlecht, H. L. Weidenmuller, J. Schmidt-Thome, J. Duphorn, and R. Tschesche. 1965. Moenomycin, a new antibiotic. II. Characterization and chemistry. Antimicrob. Agents Chemother. 5:737–742. [PubMed] [Google Scholar]
- 28.Humpert, F., F. Lalande, R. l’Hopitalier, G. Salvat, and G. Bennejean. 1991. Effect of four antibiotic additives on the Salmonella contamination of chicks protected by an adult caecal flora. Avian Pathol. 20:577–584. [DOI] [PubMed] [Google Scholar]
- 29.Joint Expert Advisory Committee on Antibiotic Resistance. 1999. The use of antibiotics in food producing animals: antibiotic resistant bacteria in animals and humans. Commonwealth Department of Health and Aged Care and Commonwealth Department of Agriculture, Fisheries and Forestry, London, United Kingdom.
- 30.Karaivanov, L., P. Koleva, M. Bonovska, M. Mateev, and A. Kozarev. 1980. Attempt at eliminating the multiple drug resistance of E. coli in pigs with enteritis using Rimactin. Vet. Med. Nauki 17:31–37. [PubMed] [Google Scholar]
- 31.Kaukas, A., and M. Hinton. 1988. The influence of the growth promoting antibiotic mupirocin on the enterococci of fattening pigs. Br. Vet. J. 144:302–309. [DOI] [PubMed] [Google Scholar]
- 32.Kruse, H., B. K. Johansen, L. M. Rorvik, and G. Schaller. 1999. The use of avoparcin as a growth promoter and the occurrence of vancomycin-resistant Enterococcus species in Norwegian poultry and swine production. Microb. Drug Resist. 5:135–139. [DOI] [PubMed] [Google Scholar]
- 33.Langlois, B. E., K. A. Dawson, I. Leak, and D. K. Aaron. 1988. Antimicrobial resistance of fecal coliforms from pigs in a herd not exposed to antimicrobial agents for 126 months. Vet. Microbiol. 18:147–153. [DOI] [PubMed] [Google Scholar]
- 34.Lebek, G. 1971. Die wirkung von flavomycin auf episomal resistente Keime. Zentbl. Vet. Med. Reihe B 19:532–539. [PubMed] [Google Scholar]
- 35.London, N., R. Nijsten, A. E. van den Bogaard, and E. E. Stobberingh. 1994. Carriage of antibiotic-resistant Escherichia coli by healthy volunteers during a 15-week period. Infection 22:187–192. [DOI] [PubMed] [Google Scholar]
- 36.Mitsuhashi, S., M. Inoue, and S. Masuyoshi. 1970. Preferential inhibition of the growth of Escherichia coli strains carrying episomes. J. Antibiot. 23:319–323. [DOI] [PubMed] [Google Scholar]
- 37.Molitoris, E., D. J. Fagerberg, C. L. Quarles, and M. I. Krichevsky. 1987. Changes in antimicrobial resistance in fecal bacteria associated with pig transit and holding times at slaughter plants. Appl. Environ. Microbiol. 53:1307–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijsten, R., N. London, A. van den Bogaard, and E. Stobberingh. 1996. Antibiotic-resistance among Escherichia coli isolated from fecal samples of pig farmers and pigs. J. Antimicrob. Chemother. 37:1131–1140. [DOI] [PubMed] [Google Scholar]
- 40.Nijsten, R., N. London, A. van den Bogaard, and E. Stobberingh. 1993. Antibiotic-resistance of enterobacteriaceae isolated from the fecal flora of fattening pigs. Vet. Q. 15:152–156. [DOI] [PubMed] [Google Scholar]
- 41.Paik, J., I. Kern, R. Lurz, and R. Hakenbeck. 1999. Mutational analysis of the Streptococcus pneumoniae bimodular class A penicillin-binding proteins. J. Bacteriol. 181:3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Select Committee on Science and Technology. 1998. Resistance to antibiotics and other antimicrobial agents. Report to the House of Lords. 7th report. The House of Lords, London, United Kingdom.
- 43.Singleton, P., and D. Sainsbury. 1993. Dictionary of microbiology and molecular biology, 2nd ed. John Wiley & Sons, Chichester, United Kingdom.
- 44.Smith, H., and J. F. Williams Tucker. 1975. The effect of feeding diets containing permitted antibiotics on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. 72:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokol, A., F. Federic, V. Kremery, V. Rejtar, and J. Janouskova. 1973. Inhibitory effect of flavomycin as a feed additive on R factors in Escherichia coli isolated from pig weanlings, p.176–179. In Proceedings of the 8th International Congress on Chemotherapy in Athens. International Society of Chemotherapy, London, United Kingdom.
- 46.Sokol, A., V. Kremery, F. Federic, V. Rejtar, and J. Janouskova. 1973. The influence of flavomycin on the elimination of R factors of Escherichia coli in vitro. Folia Microbiol. 18:176–179. [Google Scholar]
- 47.SOU Commission on Antimicrobial Feed Additives. 1997. Antimicrobial feed additives 132. Ministry of Agriculture, Stockholm, Sweden.
- 48.Spring, W. G. 1975. Extrachromosomal resistance and its control with Flavomycin. Tierarztl. Umsch. 30:591–596, 598. (In German.) [Google Scholar]
- 49.Spring, W. G. 1978. Influence of Flavomycin (bambermycin) on Salmonella infection and antibiotic resistance in pigs. Tierarztl. Umsch. 33:594, 596–597, 599. (In German.) [Google Scholar]
- 50.Spring, W. G. 1978. Influence of Flavomycin on Salmonella infection and antibiotic resistance in calves. Tierarztl. Umsch. 33:467–468, 470. (In German.) [Google Scholar]
- 51.Stutz, M. W., and G. C. Lawron. 1984. Effects of diet and antimicrobials on growth, feed efficiency, intestinal Clostridium perfringens, and ileal weight of broiler chicks. Poultry Sci. 63:2036–2042. [DOI] [PubMed] [Google Scholar]
- 52.Subramaniam-Niehaus, B., T. Schneider, J. W. Metzger, and W. Wohlleben. 1997. Isolation and analysis of moenomycin and its biosynthetic intermediates from Streptomyces ghanaensis (ATCC 14672) wildtype and selected mutants. Z. Naturforsch. 52:217–226. [DOI] [PubMed] [Google Scholar]
- 53.van den Bogaard, A., N. London, and E. E. Stobberingh. 2000. Antimicrobial resistance in pig faecal samples from The Netherlands (five abattoirs) and Sweden. J. Antimicrob. Chemother. 45:663–671. [DOI] [PubMed] [Google Scholar]
- 54.van den Bogaard, A. E., N. Bruinsma, and E. E. Stobberingh. 2000. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:146–148. [DOI] [PubMed] [Google Scholar]
- 55.van den Bogaard, A. E., P. Mertens, N. H. London, and E. E. Stobberingh. 1997. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J. Antimicrob. Chemother. 40:454–456. [DOI] [PubMed] [Google Scholar]
- 56.van den Bogaard, A. E., and E. E. Stobberingh. 1999. Antibiotic usage in animals—impact on bacterial resistance and public health. Drugs 58:589–607. [DOI] [PubMed] [Google Scholar]
- 57.van der Waaij, D. 1982. Colonization resistance of the digestive tract: clinical consequences and implications. J. Antimicrob. Chemother. 10:263–270. [DOI] [PubMed] [Google Scholar]
- 58.van der Waaij, D. 1987. Colonization resistance of the digestive tract—mechanism and clinical consequences. Nahrung. 31:507–517. [DOI] [PubMed] [Google Scholar]
- 59.Vanderwel, D., and E. E. Ishiguro. 1984. Properties of cell wall peptidoglycans synthesized by amino acid deprived ra1 mutants of Escherichia coli. Can. J. Microbiol. 30:1239–1246. [DOI] [PubMed] [Google Scholar]
- 60.van Heienoort, Y., M. Leduc, H. Singer, and J. van Heienoort. 1987. Effects of moenomycin on Escherichia coli. J. Gen. Microbiol. 133:667–674. [DOI] [PubMed] [Google Scholar]
- 61.Vollaard, E. J., and H. A. L. Clasener. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wasielewski, E., R. Mushaweck, and E. Schuetze. 1966. Moenomycin, a new antibiotic. III. Biological properties. Antimicrob. Agents Chemother. 6:743–748. [PubMed] [Google Scholar]
- 63.Williamson, R., L. Gutmann, T. Horaud, F. Delbos, and J. F. Acar. 1986. Use of penicillin-binding proteins for the identification of enterococci. J. Gen. Microbiol. 132:1929–1937. [DOI] [PubMed] [Google Scholar]
- 64.Witte, W., I. Klare, and G. Werner. 1999. Der einsatz von avoparcin als leistungsforderer und die resistenzentwicklung gegen glykopeptide bei enterokokken. Tierarztl. Praxis 27:310–315. [Google Scholar]