Abstract
The susceptibilities of 13 clinical isolates of Scedosporium apiospermum and 55 clinical isolates of S. prolificans to new and conventional drugs belonging to three different classes of antifungal agents, the azoles (miconazole, itraconazole, voriconazole, UR-9825, posaconazole), the polyenes (amphotericin B, nystatin and liposomal nystatin), and allylamines (terbinafine), were studied by use of proposed standard M38-P of NCCLS. Low growth-inhibitory antifungal activities were found in vitro for most of the drugs tested against S. prolificans isolates, with the MICs at which 90% of isolates are inhibited (MIC90s) being >8 μg/ml; the MIC90s of voriconazole and UR-9825, however, were 4 μg/ml. S. apiospermum isolates were more susceptible in vitro, with the highest activity exhibited by voriconazole (MIC90s, 0.5 μg/ml), followed by miconazole (MIC90s, 1 μg/ml), UR-9825 and posaconazole (MIC90s, 2 μg/ml), and itraconazole (MIC90s, 4 μg/ml). The MICs of terbinafine, amphotericin B, and the two formulations of nystatin (for which no statistically significant differences in antifungal activities were found for the two species) for S. apiospermum isolates were high. Cross-resistance was observed among all the azoles except posaconazole and among all the polyenes except the lipid formulation. A distribution analysis was performed with the MICs of each drug and for each species. Bimodal and skewed MIC distributions were obtained, and cutoffs indicating the borders of different MIC subpopulations of the distributions were determined on the basis of the normal plot technique. These cutoffs were in many cases reproducible between 48 and 72 h.
Scedosporium is a ubiquitous filamentous fungus with a worldwide distribution (30). The genus includes two medically important species, Scedosporium apiospermum (which is also known by its teleomorphic name Pseudallescheria boydii) and S. prolificans (which is also known as S. inflatum). S. apiospermum has been recovered from soil, sewage, polluted water (34), and animal and bird manure, while S. prolificans has been isolated from soil, cats, horses, and birds (38). These two species can easily be differentiated from each other macroscopically because of the more rapid growth of S. prolificans on potato-dextrose agar and microscopically because S. prolificans forms annellides with swollen bases (32).
These species cause infections in both immunocompetent and immunosuppressed individuals. S. prolificans can cause asymptomatic colonization of the external ear or the respiratory tract; localized infections of the joints, nail, eye, and sphenoid sinus (21); and disseminated infections in immunocompromised patients (10) with hematological malignancies (20, 31) or in organ transplant recipients (4). Similarly, S. apiospermum colonizes the tracheobronchial tree and causes mycetoma, invasive pulmonary infection (26), and disseminated infections of the central nervous system (11, 13, 14, 17).
The fungal spores infect an individual via the respiratory tract, ulcerative lesions in the gastrointestinal tract, surgical wounds, and inoculation from trauma (4). After the spores reach the target organ, they produce hyphae that may eventually sporulate in tissue, enter the bloodstream, and disseminate to other organs, particularly the kidneys, lungs, and brains (4). Most of the disseminated infections are fatal, despite antifungal treatment. If patients recover their granulocytes, they may survive.
The therapeutic approach for patients with S. apiospermum or S. prolificans infections involves complete surgical resection of the lesion with or without antifungal therapy, whose role is uncertain (12, 37). Infections caused by S. apiospermum have been treated with amphotericin B (35), miconazole, ketoconazole (38), itraconazole (29), and voriconazole (11), with variable clinical responses. S. prolificans is resistant in vitro to many antifungals, with the MICs of most of drugs tested being greater than 16 g/ml (8).
Given the demand for more aggressive antifungal therapy for the management of infections caused by Scedosporium species and the in vitro resistance of Scedosporium species to the common antifungal agents, new, more active antifungal drugs are required. Therefore, the susceptibilities of 13 clinical isolates of S. apiospermum and 55 clinical isolates of S. prolificans to new drugs (voriconazole, UR-9825, terbinafine, posaconazole, and liposomal nystatin) were determined and compared with the activities of conventional antifungal agents (miconazole, itraconazole, amphotericin B, and nystatin) (3, 9, 18). Cross-resistance between the drugs was studied, and a distribution analysis was performed in order to establish cutoffs in the in vitro susceptibilities on the basis of the frequency distributions of the MICs.
MATERIALS AND METHODS
Isolates.
A set of 68 clinical isolates of Scedosporium spp. was evaluated in the present study. The fungal isolates included 55 isolates of S. prolificans (21 from blood cultures, 10 from respiratory secretions, 3 from synovial fluid specimens, 10 from skin, ear, and superficial ulcers, 1 from a cerebrospinal fluid specimen, 2 from tissue specimens, and 8 from unspecified locations) and 13 isolates of S. apiospermum (5 from respiratory secretions, 2 from tissue specimens, 2 from wounds, and 4 from unspecified locations). The isolates were kept in 50% glycerol in water for 1 to 7 years at −70°C. Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used as quality controls.
The MICs for the isolates were determined by a microdilution method in sterile, flat-bottom, 96-well microtitration plates according to proposed standard M-38P of NCCLS for conidium-forming filamentous fungi (25).
Antifungal drugs.
Nine antifungal drugs belonging to three different classes of antifungal agents were tested in the present study, namely, the azoles miconazole and itraconazole (Janssen Research Foundation, Beerse, Belgium), UR-9825 (Uriach, Madrid, Spain), voriconazole (Pfizer Central Research, Sandwich, United Kingdom), and posaconazole (Schering-Plough Research Institute, Kenilworth, N.J.); the polyenes amphotericin B deoxycholate (Bristol-Myers Squibb, Woerden, The Netherlands) and nystatin and liposomal nystatin (Aronex Pharmaceutical, Inc., Woodlands, Tex.); and the allylamine terbinafine (Novartis, Basel, Switzerland). Miconazole, UR-9825, and nystatin were dissolved in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany), terbinafine was dissolved in DMSO with 5% Tween 80 (Merck), itraconazole was dissolved in 0.1 M HCl-100% acetone (1:1; Merck), voriconazole was dissolved in sterile distilled water with 10% dimethylformamide (Merck), and liposomal nystatin was dissolved in sterile distilled water; all solutions had final drug concentrations of 3,200 μg/ml. Liposomal nystatin was adjusted on the basis of the content of active nystatin (liposomal nystatin contains 10% [wt/wt] pure nystatin). Posaconazole was dissolved in DMSO at a final concentration of 1,600 μg/ml, and amphotericin B was dissolved in sterile distilled water at a final concentration of 5,000 μg/ml. Each drug was serially diluted twofold according to the dilution scheme of NCCLS for water-insoluble and water-soluble drugs. The drug concentration in the medium was twice the final concentration, which ranged from 64 to 0.06 μg/ml for miconazole; 32 to 0.03 μg/ml for terbinafine, itraconazole, UR-9825, nystatin, liposomal nystatin, and voriconazole; 16 to 0.015 μg/ml for amphotericin B; and 8 to 0.007 μg/ml for posaconazole. Wells 1 to 11 contained the series of drug dilutions in 100-μl volumes, and well 12 contained drug-free medium, which served as the growth control for each drug and its solvent at the final concentration used. The microtitration plates were kept at −70°C until the day of the experiments.
Medium.
The medium used was liquid RPMI 1640 medium (with ℒ-glutamine but without sodium bicarbonate; GIBCO BRL, Life Technologies, Woerden, The Netherlands) supplemented with 0.165 M MOPS (morpholinepropanesulfonic acid) buffer (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The pH of the medium was adjusted to 7.0 ± 0.1 at 22°C.
Inoculum preparation.
Each isolate had been grown for 7 days on Sabouraud dextrose agar with 0.5% chloramphenicol at room temperature and was then subcultured on the same medium for a further 5 to 7 days at 30°C. Conidia were collected with a swab and suspended in sterile water. After the heavy particles were allowed to settle, the turbidities of the supernatants were measured spectrophotometrically at 530 nm (Spectronic 20D; Milton Roy, Rochester, N.Y.), and the transmittance was adjusted to 68 to 70% by dilution. Each suspension was diluted 1:50 in RPMI 1640 medium to obtain two times the final inoculum size, which ranged from 0.5 × 104 to 5 × 104 CFU/ml. The inoculum size was verified by plating of 100 μl of serial dilutions of each inoculum onto a Sabouraud dextrose agar plate and incubation until growth became visible.
For the quality controls, the transmittances of the blastoconidial suspensions obtained from 1- to 2-day-old colonies were adjusted to 75 to 77% at 530 nm. The suspensions were diluted 1/1,000, and a final inoculum that ranged from 0.5 × 103 to 2.5 × 103 CFU/ml was used.
Incubation and MIC determination.
On the day of the test, microtitration plates were defrosted, and each well was inoculated with 100 μl of the inoculum suspension. After agitation for 15 s, the microtitration plates were incubated for 48 h at 37°C, and then incubation was continued to 72 h at 37°C. The MICs were determined visually with the aid of a concave mirror and were the lowest concentrations that showed 75% (for Scedosporium strains) or 80% (for quality controls) visible growth inhibition compared with the growth of the drug-free control. For amphotericin B, nystatin, and liposomal nystatin, the MICs were the lowest concentration that showed complete inhibition of visible growth.
Data analysis. (i) Raw data.
The high and low off-scale MICs were included in the analysis by converting the MIC to the next higher and the next lower drug concentrations, respectively. The ranges and the geometric means of the MICs were determined for each species and drug after 48 and 72 h of incubation. Furthermore, the MIC at which 90% of isolates are inhibited (MIC90) and MIC50 were calculated by use of the criteria for MIC determinations described above. For each drug the differences in the MICs after 48 and 72 h of incubation were analyzed by the nonparametric Friedman test, followed by Dunn’s multiple comparison test, and the differences were considered statistically significant when P values were smaller than 0.05. The presence of cross-resistance among the antifungal drugs after 72 h of incubation was tested by analyzing the MICs of each pair of antifungal drugs by the Spearman rank correlation, and the cross-resistance was considered statistically significant when P values were smaller than 0.01.
(ii) Distributional analysis.
In order to approximate a normal distribution, the MICs were transformed to log2 values. The frequency of each MIC was calculated for each drug and species after 48 and 72 h of incubation, and frequency distribution plots were constructed. Deviations for the normal distribution were analyzed by the Shapiro-Wilk W test (SPSS version 9.0, SPSS Inc., Chicago, Ill.), and the correlation coefficient W between the distribution of the log2 MICs of each drug and the normal distribution was reported. A correlation coefficient W equal to 1 indicated the perfect compatibility of the data with the normal distribution. A P value smaller than 0.05 indicated a statistically significant deviation. The correlation coefficient assesses the straight-line association between the observed values and the expected values derived from the normal distribution on the basis of Blom’s proportional estimation formula (1). In order to analyze the deviation further, the normal plot technique was used, and the presence of more than one distribution in the data was checked (1). One population of data that follows a normal distribution is represented by a straight line in the normal plot. Thus, different slopes in these plots indicated different MIC groups. On the basis of the normal plots, the cutoff between MIC subpopulations was determined graphically as the lowest drug concentration after which the slope changed. Cutoffs were not determined for normal plots with less than three datum points.
RESULTS
The susceptibilities of two quality control isolates, C. krusei (ATCC 6258) and C. parapsilosis (ATCC 22019), to the nine antifungal drugs tested are presented in Table 1. The isolates were tested up to three times, and the MIC ranges are reported.
TABLE 1.
Susceptibilities of quality control strains to various antifungal drugs after 48 h of incubation, based on 100% inhibition of growth for polyenes and 80% for the other drugsa
| Antifungal agent | MIC range (μg/ml)
|
|
|---|---|---|
| C. krusei (ATCC 6258) | C. parapsilosis (ATCC 22019) | |
| Miconazole | 1–2 | 1–2 |
| Terbinafine | >32 | 0.125–0.5 |
| Itraconazole | 0.5 | 0.25–0.5 |
| UR-9825 | 0.06–0.125 | <0.03 |
| Voriconazole | 0.125–0.25 | 0.03–0.06 |
| Amphotericin B | 0.5–1 | 0.25–0.5 |
| Posaconazole | 0.25–0.5 | 0.125–0.25 |
| Nystatin | 2–4 | 2–4 |
| Liposomal nystatin | 2–4 | 1–2 |
The isolates were tested in triplicate.
The results of in vitro susceptibility testing of 55 S. prolificans strains are shown in Table 2. The MICs of the new and conventional antifungal drugs used in the present study for S. prolificans were very high, with the MIC90s of all drugs except for those of voriconazole and UR-9825 exceeding 16 μg/ml after 72 h of incubation; those of voriconazole and UR-9825 were 4 μg/ml after 72 h of incubation. Nevertheless, the terbinafine and amphotericin B MICs (2 μg/ml) for some strains were relatively low (10-fold lower than the geometric mean MIC). A statistically significant elevation in the MIC after 72 h was observed only for terbinafine (P < 0.01), for which there was an increase in the geometric mean MIC after 72 h of more than twofold. The large number of off-scale MICs did not allow the performance of the Spearman rank correlation to check for cross-resistance among the antifungal drugs.
TABLE 2.
Antifungal activities of conventional and new antifungal drugs against 55 clinical S. prolificans isolates
| Incubation period and antifungal | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | Geometric mean | 50% | 90% | |
| 48 h | ||||
| Miconazole | 1–16 | 5.98 | 8 | 16 |
| Terbinafine | 1–32 | 8.34 | 8 | 16 |
| Itraconazole | 2–>32 | >32 | >32 | >32 |
| UR-9825 | 0.5–4 | 1.69 | 2 | 2 |
| Voriconazole | 0.5–8 | 2.79 | 4 | 4 |
| Amphotericin B | 1–>16 | 15.34 | 16 | >16 |
| Posaconazole | 2–>8 | >8 | >8 | >8 |
| Nystatin | 8–32 | 20.35 | 16 | 32 |
| Liposomal nystatin | 8–32 | 19.23 | 16 | 32 |
| 72 h | ||||
| Miconazole | 4–>64 | 9.85 | 8 | 64 |
| Terbinafine | 2–>32 | 19.16 | 16 | 32 |
| Itraconazole | >32 | >32 | >32 | >32 |
| UR-9825 | 0.5–8 | 1.97 | 2 | 4 |
| Voriconazole | 1–8 | 3.29 | 4 | 4 |
| Amphotericin B | 2–>16 | >16 | >16 | >16 |
| Posaconazole | >8 | >8 | >8 | >8 |
| Nystatin | 16–>32 | 32 | 32 | >32 |
| Liposomal nystatin | 16–32 | 32 | 32 | 32 |
The MICs for S. apiospermum were lower than those for S. prolificans (Table 3). Low growth-inhibitory activities were found for liposomal nystatin, nystatin, and terbinafine, the MIC90s of which were greater than 8 μg/ml after 72 h. The highest activity was obtained for voriconazole (MIC90s, 0.5 μg/ml). High levels of activity were also found for miconazole (MIC90, 1 μg/ml), posaconazole (MIC90, 2 μg/ml), and UR-9825 (MIC90, 2 μg/ml). Itraconazole and amphotericin B were less active since the MIC90s were higher (4 and 16 μg/ml, respectively). No statistically significant differences in the activities after 48 and 72 h of incubation were observed for any of the drugs. The activity of liposomal nystatin was not statistically significantly different from the activity of nystatin after both 48 and 72 h of incubation. A statistically significant correlation between the activities of nystatin and liposomal nystatin was found by Spearman rank correlation analysis (Spearman rank correlation coefficient [rs] = 0.92) as well as among all azoles except posaconazole (for voriconazole, itraconazole, UR-9825, and miconazole, rs > 0.84; for posaconazole, rs < 0.54). Furthermore, a statistically significant correlation was found between amphotericin B and nystatin (rs = 0.77) but not liposomal nystatin (rs = 0.49) (Table 4).
TABLE 3.
Antifungal activities of conventional and new antifungal drugs against 13 clinical S. apiospermum isolates
| Incubation period and antifungal | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | Geometric mean | 50% | 90% | |
| 48 h | ||||
| Miconazole | 0.125 | 0.34 | 0.25 | 1 |
| Terbinafine | 2–>32 | 32 | 32 | >32 |
| Itraconazole | 0.03–2 | 0.35 | 0.5 | 2 |
| UR-9825 | 0.03–1 | 0.13 | 0.125 | 1 |
| Voriconazole | 0.03–0.5 | 0.09 | 0.125 | 0.25 |
| Amphotericin B | 0.5–8 | 1.72 | 2 | 4 |
| Posaconazole | 0.125–1 | 0.42 | 0.5 | 1 |
| Nystatin | 2–16 | 5.99 | 4 | 16 |
| Liposomal nystatin | 2–16 | 5.99 | 4 | 16 |
| 72 h | ||||
| Miconazole | 0.25–1 | 0.58 | 0.5 | 1 |
| Terbinafine | 32–>32 | >32 | >32 | >32 |
| Itraconazole | 0.25–8 | 0.78 | 0.5 | 4 |
| UR-9825 | 0.06–2 | 0.41 | 0.5 | 2 |
| Voriconazole | 0.03–0.5 | 0.17 | 0.25 | 0.5 |
| Amphotericin B | 1–16 | 2.97 | 4 | 16 |
| Posaconazole | 0.25–2 | 0.79 | 1 | 2 |
| Nystatin | 4–32 | 12.70 | 16 | 32 |
| Liposomal nystatin | 4–16 | 11.99 | 16 | 16 |
TABLE 4.
Spearman rank correlation coefficients between the MICs of antifungal drugs for 13 clinical S. apiospermum isolates after 72 h of incubationa
| Drug group and drug | Correlation coefficient
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Azoles
|
Polyenes
|
|||||||
| MCZ | ICZ | UR | VCZ | PCZ | AMB | NYS | L-NYS | |
| Azoles | ||||||||
| MCZ | 1.00 | 0.88b | 0.84b | 0.84b | 0.44 | 0.40 | 0.68 | 0.34 |
| ICZ | 1.00 | 0.85b | 0.85b | 0.50 | 0.46 | 0.66 | 0.49 | |
| UR | 1.00 | 0.97b | 0.54 | 0.25 | 0.69 | 0.46 | ||
| VCZ | 1.00 | 0.51 | 0.33 | 0.65 | 0.25 | |||
| PCZ | 1.00 | 0.03 | 0.57 | 0.52 | ||||
| Polyenes | ||||||||
| AMB | 1.00 | 0.77b | 0.49 | |||||
| NYS | 1.00 | 0.92b | ||||||
| L-NYS | 1.00 | |||||||
MCZ, miconazole; ICZ, itraconazole; UR, UR-9825; VCZ, voriconazole; PCZ, posaconazole; AMB, amphotericin B; NYS, nystatin; L-NYS, liposomal nystatin.
P < 0.01.
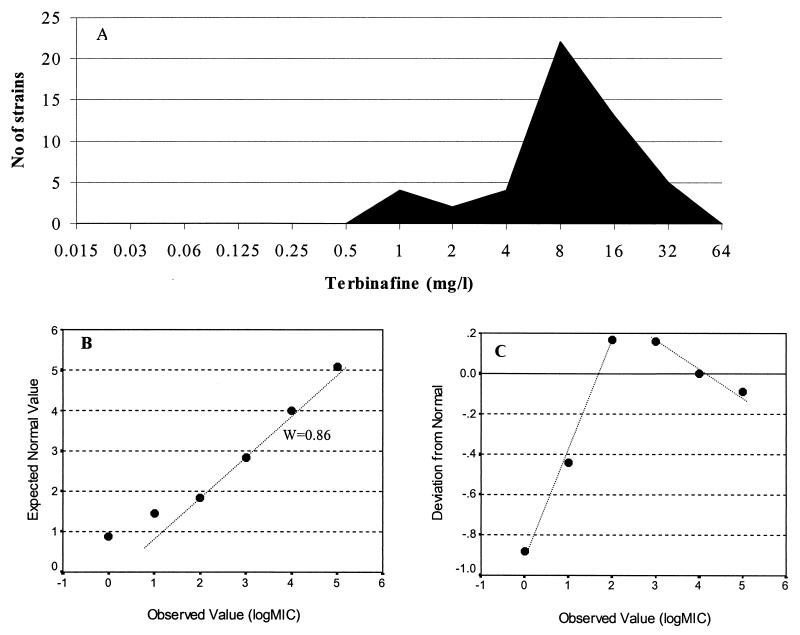
Various types of frequency distribution plots characterized by the presence of two peaks and a skewness toward lower or higher drug concentrations were obtained. The correlation coefficients obtained by the Shapiro-Wilk W test ranged from 0.29 to 0.95, showing statistically significant deviations from the normal distribution especially for S. prolificans. However, by analysis of the normal plots of these distributions, two different groups of MICs were found. These were characterized by the different standard deviations suggested by the slopes in the normal plots even when the deviation from the normal distribution was not significant (Fig. 1). In many cases reproducible cutoffs between subpopulations were determined after 48 and 72 h of incubation, although the cutoffs were different for the two species. The results are summarized in Table 5. For S. prolificans after 72 h of incubation, the cutoffs in the normal plots were as follows: 16 μg/ml for miconazole, terbinafine, and nystatin; 8 μg/ml for amphotericin B; 4 μg/ml for UR-9825; and 2 μg/ml for voriconazole. For S. apiospermum after 72 h of incubation the cutoffs in the normal plots were as follows: 0.25 μg/ml for miconazole, UR-9825, and posaconazole; 0.125 μg/ml for voriconazole; 1 μg/ml for itraconazole; 2 μg/ml for amphotericin B; and 8 μg/ml for the two formulations of nystatin.
FIG. 1.
(A) Frequency distribution plot of the MICs of terbinafine for S. prolificans strains after 48 h of incubation. (B) Observed log2 MICs plotted against the expected log2 MICs derived from the normal distribution based on Blom’s proportion estimation formula. (C) Normal plots constructed by plotting the observed log2 MICs against the normal scores (number of standard deviations below or above the mean of expected values). The normal plot revealed a mixture of two distributions in the data represented by the different slopes, which indicated the different standard deviations in the two putative groups. These two groups may have different in vitro susceptibilities, and therefore, a cutoff of 4 μg/ml was determined.
TABLE 5.
Cutoffs in distributions of MICs determined from the normal plots
| Antifungal | MIC cutoff (μg/ml)a
|
|||
|---|---|---|---|---|
|
S. prolificans
|
S. apiospermum
|
|||
| 48 h | 72 h | 48 h | 72 h | |
| Miconazole | 4 (0.83) | 16 (0.77) | 0.25 (0.87b) | 0.25 (0.83) |
| Terbinafine | 4 (0.86) | 16 (0.88) | 16 (0.60) | NDc |
| Itraconazole | 32 (0.29) | ND | 0.25 (0.94b) | 1 (0.89b) |
| UR-9825 | 2 (0.79) | 4 (0.81) | 0.06 (0.85) | 0.25 (0.95b) |
| Voriconazole | 1 (0.80) | 2 (0.78) | 0.06 (0.88b) | 0.125 (0.94b) |
| Amphotericin | 8 (0.84) | 8 (0.60) | 1 (0.89b | 2 (0.84) |
| Posaconazole | ND | ND | 0.25 (0.88b) | 0.25 (0.90b) |
| Nystatin | 8 (0.72) | 16 (0.61) | 4 (0.86b) | 8 (0.85) |
| Liposomal nystatin | 8 (0.69) | ND | 4 (0.80) | 8 (0.58) |
The cutoffs were determined graphically from the normal plots (the values in parentheses are the W correlation coefficients between the distribution of the MICs and the normal distribution obtained by the Shapiro-Wilk test; P < 0.05).
P > 0.05.
ND, not determined.
DISCUSSION
Since 1899, when S. apiospermum was recovered from the ear of a child with chronic otitis externa (33), and 1984, when the first case of S. prolificans infection was described in a man with osteomyelitis (19), many reports of infections caused by these species have followed (4, 12, 35). The clinical spectra of these infections may be similar (37, 38), with localized infections occurring in immunocompetent hosts and dissemination more likely occurring in immunocompromised individuals (28). The optimal antifungal therapy for these infections is unknown. Given the in vitro resistance of the two species, especially S. prolificans, to most of the conventional antifungal drugs, the use of agents with greater activities against these species is warranted. Therefore, the antifungal activities of nine antifungal drugs including new and conventional agents belonging to three different classes of antifungal drugs (five azoles, three polyenes, and one allylamine) against 55 clinical isolates of S. prolificans and 13 clinical isolates of S. apiospermum were studied by using the proposed M38-P standard of NCCLS, based on a 100% reduction of growth for polyenes and a 75% reduction of growth for all the other agents.
Most of the antifungal agents were found to have poor activities against the S. prolificans strains tested since the MIC90s were higher than 8 μg/ml. The most active drugs were the new antifungal agents UR-9825 and voriconazole since these drugs inhibited the growth of most of the strains at 4 μg/ml, respectively. S. apiospermum was more susceptible, with the highest levels of activity against S. apiospermum exhibited by voriconazole and miconazole (MIC90s, 0.5 and 1 μg/ml, respectively), followed by posaconazole and UR-9825 (MIC90s, 2 μg/ml) and itraconazole (MIC90s, 4 μg/ml).
These results are in agreement with those of a previous study in which the resistance of 43 clinical isolates of S. prolificans and the relative susceptibilities of 23 clinical S. apiospermum isolates to various antifungal drugs were reported (8). In that study higher MICs were found for miconazole (MICs > 16 μg/ml) since complete inhibition of growth was chosen for determination of the antifungal activities of azoles, whereas in the present study 75% inhibition of growth was used. By use of this MIC endpoint, trailing phenomena by azoles which may influence the results are eliminated and a high degree of reproducibility was achieved (22). Furthermore, more information about the activities of antifungal drugs was obtained since any effect of the antifungal drugs on the growth of fungi can be observed and a low level of activity can be differentiated from the absence of any antifungal activity. When complete inhibition of growth is chosen as the criterion for determination of the MIC, this information is missed, especially when complete inhibition is not apparent in any of the drug dilutions used. This aspect might be very important when one is looking for combinations of antifungal drugs with synergistic activities, since even a low level of activity of an agent can be enhanced when the agent is combined with another agent (24).
From this point of view, among the antifungal drugs which showed high MICs, antifungal effects were found for miconazole, terbinafine, and nystatin formulations but not for itraconazole, posaconazole, or amphotericin B for most of the strains of S. prolificans tested. In a recent study, the antifungal activity of terbinafine was enhanced when it was combined with itraconazole, resulting in a strong synergistic interaction against 20 clinical S. prolificans isolates (24).
The two formulations of nystatin tested in the present study did not show statistically significantly different in vitro activities. This is in agreement with the results of a previous study, in which no difference in the activities of the two nystatin formulations against these two Scedosporium species was described (7). In other studies, liposomal nystatin showed higher levels of activity against Aspergillus species (16, 27), although for one species, Aspergillus terreus, the MICs of liposomal nystatin were higher than those of nystatin. The overall higher MICs of lipid formulations of amphotericin B compared with those found for conventional formulations of amphotericin B were linked to the incomplete and variable release of active amphotericin B from liposomes. Opposite effects were explained by the production of phospholipases by the fungi, an event which increases the level of release of the active drug from the liposomes and therefore the activity of the formulation (6). Thus, in liposomal nystatin the interaction of nystatin with the lipids might be less stable than the corresponding interaction in lipid formulations of amphotericin B, and therefore, the release of active drug is easier, resulting in the same activity with the pure drug or a higher level of activity when phospholipases are produced by fungi.
The on-scale MICs for the S. apiospermum isolates tested allowed the search for cross-resistance between the antifungal agents by correlating the MICs. A high level of correlation was found between nystatin and liposomal nystatin as well as between amphotericin B and nystatin, although the correlation for the latter was less strong, indicating the similarities in their mechanisms of action. However, no statistically significant correlation was found between amphotericin B and liposomal nystatin, since the different formulations of these two compounds may influence the mechanism of action (6). Cross-resistance was found among all azoles except posaconazole, which suggests that the mechanism of action or the mechanism of resistance for posaconazole might be different from those for the other azoles.
On the basis of the in vitro data obtained in the present study, the most effective drugs against S. prolificans are UR-9825 and voriconazole. Unfortunately, there are no reports in the literature of treatment with these drugs to confirm their efficacies in vivo. On the contrary, there are reports of successful treatment of localized infections with amphotericin B, miconazole, ketoconazole, flucytosine (37), and fluconazole (28), as well as reports of successful treatment of infections in neutropenic patients with itraconazole (5, 15, 38) and amphotericin B, miconazole, and fluconazole (30, 38), despite the apparent in vitro resistance of the organism to these antifungal drugs. However, serious consideration must be given to the fact that the primary reason for the recovery of those patients may not be the antifungal therapy but additional surgical treatment or the recovery of host defenses in the patients (15, 37) and the administration of granulocyte-macrophage-stimulating factors (5, 38). It is also possible that some drugs such as itraconazole can prevent the development of invasive infection, as was described for an AIDS patient (15), and can prolong survival in a nonneutropenic murine model of invasive disseminated scedosporiosis (unpublished data).
For S. apiospermum the most effective drugs in vitro were voriconazole, posaconazole, miconazole, itraconazole, and UR-9825. This has been confirmed by many reports in the literature of successful treatment with the drugs mentioned above except UR-9825 and posaconazole; no reports of treatment of clinical cases of infection with UR-9825 and posaconazole have been published. UR-9825 and posaconazole might be attractive antifungal therapies, given their high levels of in vitro activity. Intravenous administration of miconazole, despite the positive results even for patients whose immune systems were impaired (34), had significant drawbacks such as hypotension, pruritus, and bone marrow and hepatic toxic effects (26). In addition, there were high rates of infection recurrence in those treated with miconazole (29). Itraconazole and voriconazole, for which oral and intravenous formulations are available and which have fewer side effects than miconazole, might be alternatives to miconazole for the treatment of S. apiospermum infections (29).
Since no breakpoints for filamentous fungal infections have yet been established, conclusions regarding the interpretation of the MICs cannot be made. However, the distribution of the MICs of each drug may give information on the presence within the collections tested of subpopulations with different in vitro susceptibilities. Based on the normal plot technique, cutoffs between the subpopulations were determined for each drug and species. In many cases these were reproducible within the two incubation periods. However, the importance of these results should be evaluated in animal models in an attempt to correlate the in vitro data with the in vivo outcome.
The high fatality rates from infections caused by S. apiospermum and S. prolificans (2) and the increasing numbers of infections caused by these species impose the necessity for new approaches to the antifungal therapy used to treat these infections. The development of new drugs more effective against these species or combination therapy (23, 24, 36) may contribute to the management of invasive Scedosporium infections.
Acknowledgments
This work was supported by European Commission Training and Mobility of Researchers grant FMRX-CT970145 (to Joseph Meletiadis) and by the Mycology Research Center of Nijmegen (Nijmegen, The Netherlands).
We thank B. Bouman and E. Kramer for technical assistance.
REFERENCES
- 1.Altman, D. G. 1991. Preparing to analyse data, p.132–143. In Practical statistics for medical research. Chapman & Hall/CRC, London, United Kingdom.
- 2.Alvarez, M., B. Lopez Ponga, C. Rayon, J. Garcia Gala, M. C. Roson Porto, M. Gonzalez, J. V. Martinez-Suarez, and J. L. Rodriguez-Tudela. 1995. Nosocomial outbreak caused by Scedosporium prolificans (inflatum): four fatal cases in leukemic patients. J. Clin. Microbiol. 33:3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balfour, J. A., and D. Faulds. 1992. Terbinafine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses. Drugs 43:259–284. [DOI] [PubMed] [Google Scholar]
- 4.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, and J. V. Martinez-Suarez. 1997. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Scedosporium prolificans Spanish Study Group. Medicine (Baltimore) 76:256–265. [DOI] [PubMed] [Google Scholar]
- 5.Bouza, E., P. Munoz, L. Vega, M. Rodriguez-Creixems, J. Berenguer, and A. Escudero. 1996. Clinical resolution of Scedosporium prolificans fungemia associated with reversal of neutropenia following administration of granulocyte colony-stimulating factor. Clin. Infect. Dis. 23:192–193. [DOI] [PubMed] [Google Scholar]
- 6.Carrillo-Munoz, A. J., G. Quindos, C. Tur, M. Ruesga, R. Alonso, O. del Valle, V. Rodriguez, M. P. Arevalo, J. Salgado, E. Martin-Mazuelos, F. J. Bornay-Llinares, A. del Palacio, M. Cuetara, I. Gasser, J. M. Hernandez-Molina, and J. Peman. 2000. Comparative in vitro antifungal activity of amphotericin B lipid complex, amphotericin B and fluconazole. Chemotherapy (Basel) 46:235–244. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Munoz, A. J., G. Quindos, C. Tur, M. T. Ruesga, Y. Miranda, O. del Valle, P. A. Cossum, and T. L. Wallace. 1999. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother. 44:397–401. [DOI] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149–151. [DOI] [PubMed] [Google Scholar]
- 9.Daneshmend, T. K., and D. W. Warnock. 1983. Clinical pharmacokinetics of systemic antifungal drugs. Clin. Pharmacokinet. 8:17–42. [DOI] [PubMed] [Google Scholar]
- 10.Farag, S. S., F. C. Firkin, J. H. Andrew, C. S. Lee, and D. H. Ellis. 1992. Fatal disseminated Scedosporium inflatum infection in a neutropenic immunocompromised patient. J. Infect. 25:201–204. [DOI] [PubMed] [Google Scholar]
- 11.Girmenia, C., G. Luzi, M. Monaco, and P. Martino. 1998. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 36:1436–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosbell, I. B., M. L. Morris, J. H. Gallo, K. A. Weeks, S. A. Neville, A. H. Rogers, R. H. Andrews, and D. H. Ellis. 1999. Clinical, pathologic and epidemiologic features of infection with Scedosporium prolificans: four cases and review. Clin. Microbiol. Infect. 5:672–686. [Google Scholar]
- 13.Guyotat, D., M. A. Piens, R. Bouvier, and D. Fiere. 1987. A case of disseminated Scedosporium apiospermum infection after bone marrow transplantation. Mycoses 30:151–154. [DOI] [PubMed] [Google Scholar]
- 14.Hofman, P., M. C. Saint-Paul, M. Gari-Toussaint, J. F. Michiels, C. Boissy, P. Jambou, J. Gugenheim, and R. Loubiere. 1993. Disseminated infection due to Scedosporium apiospermum in liver transplantation. Differential diagnosis from invasive aspergillosis. Ann. Pathol. 13:332–335. [PubMed] [Google Scholar]
- 15.Hopwood, V., E. G. Evans, J. Matthews, and D. W. Denning. 1995. Scedosporium prolificans, a multiresistant fungus, from a U.K. AIDS patient. J. Infect. 30:153–155. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, E. M., J. O. Ojwang, A. Szekely, T. L. Wallace, and D. W. Warnock. 1998. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob. Agents Chemother. 42:1412–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez, F. A., R. S. Crowley, L. Wastila, H. A. Valantine, and J. S. Remington. 1998. Scedosporium apiospermum (Pseudallescheria boydii) infection in a heart transplant recipient: a case of mistaken identity. J. Heart Lung Transplant. 17:321–324. [PubMed] [Google Scholar]
- 18.Lyman, C. A., and T. J. Walsh. 1992. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs 44:9–35. [DOI] [PubMed] [Google Scholar]
- 19.Malloch, D., and I. F. Salkin. 1984. A new species of Scedosporium associated with osteomyelitis in humans. Mycotaxon 21:247–255. [Google Scholar]
- 20.Marin, J., M. A. Sanz, G. F. Sanz, J. Guarro, M. L. Martinez, M. Prieto, E. Gueho, and J. L. Menezo. 1991. Disseminated Scedosporium inflatum infection in a patient with acute myeloblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 10:759–761. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto, T., L. Ajello, T. Matsuda, P. J. Szaniszlo, and T. J. Walsh. 1994. Developments in hyalohyphomycosis and phaeohyphomycosis. J. Med. Vet. Mycol. 32:329–349. [DOI] [PubMed] [Google Scholar]
- 22.Meletiadis, J., J. F. G. M. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, and P. E. Verweij. 2000. Combination chemotherapy for the treatment of invasive infections by Scedosporium prolificans. Clin. Microbiol. Infect. 6:336–337. [DOI] [PubMed] [Google Scholar]
- 24.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. G. M. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. Document M-38P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Nomdedeu, J., S. Brunet, R. Martino, A. Altes, V. Ausina, and A. Domingo-Albos. 1993. Successful treatment of pneumonia due to Scedosporium apiospermum with itraconazole: case report. Clin. Infect. Dis. 16:731–733. [DOI] [PubMed] [Google Scholar]
- 27.Oakley, K. L., C. B. Moore, and D. W. Denning. 1999. Comparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole. Antimicrob. Agents Chemother. 43:1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickles, R. W., D. E. Pacey, D. B. Muir, and W. H. Merrell. 1996. Experience with infection by Scedosporium prolificans including apparent cure with fluconazole therapy. J. Infect. 33:193–197. [DOI] [PubMed] [Google Scholar]
- 29.Piper, J. P., J. Golden, D. Brown, and J. Broestler. 1990. Successful treatment of Scedosporium apiospermum suppurative arthritis with itraconazole. Pediatr. Infect. Dis. J. 9:674–675. [PubMed] [Google Scholar]
- 30.Rabodonirina, M., S. Paulus, F. Thevenet, R. Loire, E. Gueho, O. Bastien, J. F. Mornex, M. Celard, and M. A. Piens. 1994. Disseminated Scedosporium prolificans (S. inflatum) infection after single-lung transplantation. Clin. Infect. Dis. 19:138–142. [DOI] [PubMed] [Google Scholar]
- 31.Salesa, R., A. Burgos, R. Ondiviela, C. Richard, G. Quindos, and J. Ponton. 1993. Fatal disseminated infection by Scedosporium inflatum after bone marrow transplantation. Scand. J. Infect. Dis. 25:389–393. [DOI] [PubMed] [Google Scholar]
- 32.Salkin, I. F., M. R. McGinnis, M. J. Dykstra, and M. G. Rinaldi. 1988. Scedosporium inflatum, an emerging pathogen. J. Clin. Microbiol. 26:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siebenmann, F. 1899. Die Schimmelmykosen des menschlichen Ohres, p.95. Wiesbaden, Germany.
- 34.Tekavec, J., E. Mlinaric-Missoni, and V. Babic-Vazic. 1997. Pulmonary tuberculosis associated with invasive pseudallescheriasis. Chest 111:508–511. [DOI] [PubMed] [Google Scholar]
- 35.Travis, L. B., G. D. Roberts, and W. R. Wilson. 1985. Clinical significance of Pseudallescheria boydii: a review of 10 years’ experience. Mayo Clin. Proc. 60:531–537. [DOI] [PubMed] [Google Scholar]
- 36.Verweij, P. E., N. J. Cox, and J. F. G. M. Meis. 1997. Oral terbinafine for treatment of pulmonary Pseudallescheria boydii infection refractory to itraconazole therapy. Eur. J. Clin. Microbiol. Infect. Dis. 16:26–28. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, C. M., E. J. O’Rourke, M. R. McGinnis, and I. F. Salkin. 1990. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J. Infect. Dis. 161:102–107. [DOI] [PubMed] [Google Scholar]
- 38.Wood, G. M., J. G. McCormack, D. B. Muir, D. H. Ellis, M. F. Ridley, R. Pritchard, and M. Harrison. 1992. Clinical features of human infection with Scedosporium inflatum. Clin. Infect. Dis. 14:1027–1033. [DOI] [PubMed] [Google Scholar]