Abstract
In this paper, we describe the development and use of enzymatic assays to determine intracellular lamivudine triphosphate (3TCTP) and carbovir triphosphate (CBVTP) concentrations in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus (HIV)-infected patients. The assays involve inhibition of HIV reverse transcriptase (RT), which normally incorporates radiolabeled deoxynucleoside triphosphates into a synthetic template primer. For the 3TCTP assay, a preincubation procedure was added whereby 3TCTP becomes incorporated before [3H]dCTP. At a 1:400 template primer dilution, control product formation was reduced by 88.0% with 0.8 pmol of 3TCTP. Standard 3TCTP inhibition curves were performed using this procedure. For the CBVTP assay, 0.1 pmol of CBVTP inhibited control product formation with and without the use of a preincubation step, so inhibition curves were constructed using both procedures. However, reduced template primer stability with assays using preincubation steps led to a single-incubation procedure being adopted for future studies. The presence of PBMC extracts interfered with the 3TCTP assay. However, this was overcome by the addition of CuSO4. PBMC extracts did not interfere with the CBVTP assay. Intracellular 3TCTP and CBVTP concentrations were determined in PBMCs from HIV-infected patients over 24 h or greater. Peak concentrations were obtained 6 to 8 h after dosing, and the half-lives of the anabolites suggested the possibility of once-daily dosing. These assays are currently being used for determination of 3TCTP and CBVTP concentrations in clinical studies.
Previous analytical techniques have employed a combined high-pressure liquid chromatography-radioimmunoassay (HPLC-RIA) procedure for the measurement of intracellular dideoxynucleoside (ddN) phosphates in vivo (1, 2, 8, 11, 12, 15, 16, 19, 20). However, this methodology has the problem of requiring a large volume of blood from the patient (>20 ml per time point). Furthermore, the combined HPLC-RIA method is very time-consuming, with only a small number of samples processed daily. More recently, ddN phosphates have been determined by liquid chromatography tandem mass spectrometry (LC-MS-MS) (17). While this method has many advantages, there is a requirement for specialized and expensive equipment and time-consuming sample preparation.
An alternative approach is to measure the concentrations of the active ddN triphosphate (ddNTP) using an enzymatic assay. This procedure involves the inhibition of human immunodeficiency virus (HIV) reverse transcriptase (RT) enzyme that normally incorporates radiolabeled dNTPs into a specific synthetic template primer. By using known standard amounts of the ddNTP inhibitor, an inhibition curve can be derived. Therefore, the concentrations of the respective ddNTP present in cell extracts can be determined from the standard curve.
Initial studies performed by Robbins et al. (14) using this type of procedure involved the determination of zidovudine TP (ZDVTP). Although the method was primarily used to study the mechanism and kinetics of inhibition rather than to quantify the concentration of ZDVTP in patient cell extracts, Robbins et al. (14) indicated that it might have clinical uses for monitoring ZDVTP concentrations in HIV-infected patients.
One of the main problems with these initial studies was the high degree of interference observed with the assay when peripheral blood mononuclear cell (PBMC) extracts were added to the reaction mixtures. Furthermore, the degree of inhibition observed with the standard ZDVTP inhibition curve meant the sensitivity needed to be improved.
Subsequent modifications of the general assay procedure have involved limiting the number of binding sites to which endogenous and drug TPs can bind through a reduction in the amount of template primer used in the assay (13).
In this report, we describe the development of similar procedures for the determination of lamivudine TP (3TCTP) and carbovir TP (CBVTP; the active anabolite of abacavir [ABC]) concentrations in PBMCs from HIV-infected patients. The main aims were to develop sensitive and reproducible assays requiring <10 × 106 cells while minimizing interference and to increase sample throughput compared to the combined HPLC-RIA method. Investigations into the intracellular profiles of 3TCTP and CBVTP levels in PBMCs from HIV-infected patients over time were also undertaken. Results from the CBVTP arm of this study have recently been presented (S. Kewn, B. Maher, P. G. Hoggard, S. H. Khoo, P. Carey, E. Wilkins, J. Gould, and D. J. Back, Abstr. 5th Int. Cong. Drug Ther. HIV Infect., abstr. P276A, 2000).
MATERIALS AND METHODS
Chemicals.
[5′-3H]dCTP (specific activity, 31.7 Ci mmol−1) and [5′-3H]dGTP (specific activity, 4.0 Ci mmol−1) were purchased from Moravek Biochemicals Inc., Brea, Calif. HIV RT was obtained from Amersham Pharmacia Biotech U.K. Ltd., Little Chalfont, Buckinghamshire, England (manufactured by the Research Foundation for Microbial Disease of Osaka University, Osaka, Japan). Poly(dC18) and poly(rI18) were purchased from Cruachem Ltd., Glasgow, Scotland. Poly(rC) · poly(dG)12–18 was obtained from Amersham Pharmacia Biotech Inc., Piscataway, N.J. 3TCTP (93.2% pure) and CBVTP (90.4% pure) were kindly donated by Glaxo-SmithKline, Greenford, United Kingdom. DE81 filter papers (25-mm diameter DEAE paper) were acquired from Whatman, Maidstone, United Kingdom. Liquid scintillation fluid (Ultima Gold) was obtained from Packard BioScience B.V., Groningen, The Netherlands. Lymphoprep was procured from Nycomed Pharma AS, Oslo, Norway. All other chemicals were purchased from Sigma Chemical Company Ltd., Poole, United Kingdom.
Preparation of PBMC extracts from healthy volunteers and HIV-infected patients.
Fresh heparinized venous blood (10 to 20 ml) was collected from either healthy volunteers or HIV-infected patients, and PBMCs were isolated by the method of density cushion centrifugation using lymphoprep resolving medium (9). The PBMCs were then washed in phosphate-buffered saline and counted using a hemocytometer. The cells were centrifuged (2,772 × g; 4 min; 4°C), and the resulting cell pellet was extracted overnight in 60% methanol (2 ml) at 4°C. Following extraction, the cell suspensions were centrifuged (2,772 × g; 4 min; 4°C), and the methanolic supernatant fractions were evaporated under a stream of nitrogen.
The dried down methanolic extracts were then reconstituted in perchloric acid (0.4 N; 100 μl; 4°C) and allowed to extract again for 30 min. Samples were centrifuged (2,772 × g; 4 min; 4°C), and the acid supernatant fractions (100 μl) were quantitatively transferred to fresh 1.5-ml microcentrifuge tubes.
The acid extracts were neutralized by the addition of freshly prepared 0.5 N trioctylamine (27%) in 1′,1′,2′-trichlorotrifluoroethane (73%) (100 μl) to each tube. After rotary mixing (15 min), the phases were separated by centrifugation (6,000 × g; 10 s). An aliquot (85 to 90 μl) of the upper aqueous phase (of the resulting three-phase system) was carefully aspirated into 1.5-ml microcentrifuge tubes. The resulting extracts were then checked with pH paper to ensure successful neutralization (pH 7.0). The extracts were stored (−20°C) until determination of intracellular levels was carried out.
Enzymatic assay development.
The 3TCTP and CBVTP assays are described separately.
(i) 3TCTP assay development. (a) Template primer preparation.
dC18 (67.3 nmol; 9.6 optical density units) was solubilized in 922 μl of 50 mM Tris-HCl (pH 8.0) to give a 73.0-pmol μl−1 solution, which was then further diluted to give a 36.5-pmol μl−1 solution. Likewise, 674 μl of Tris-HCl was added to rI18 (49.4 nmol; 8.5 optical density units) to give a 73.0-pmol μl−1 solution, which was then further diluted to give a 36.5-pmol μl−1 solution. To prepare the template primer itself, 20 μl of the 36.5-pmol μl−1 dC18 solution was added to 80 μl of the 36.5-pmol μl−1 rI18 solution in an Eppendorf tube. The tube was then heated to 56°C for 15 min in a heat block to allow annealing to occur. Following this period, the tube was rapidly cooled on ice and stored at −20°C until it was used. This was the stock undiluted template primer solution (the solution contained 7.3 pmol of dC18 μl−1 and 29.2 pmol of rI18 μl−1). The stock undiluted template primer solution was diluted as required with 50 mM Tris-HCl (pH 8.0).
(b) Comparison of inhibition by 3TCTP with and without a preincubation procedure.
For the single-incubation procedure, control product formation (i.e., incorporation of [3H]dCTP into the template primer in the absence of inhibitor) was determined using a reaction mixture containing [3H]dCTP (24 pmol), 5 μl of undiluted or 1:40 or 1:400 dilution of template primer, 30 mM KCl, 0.1% (wt/vol) Triton X-100, 0.025% (wt/vol) bovine serum albumin (BSA), 0.5 mM EDTA, 1 mM dithiothreitol, 6 mM MgCl2, 50 mM Tris-HCl (pH 8.0), and 0.4 U of HIV RT. The total mixture volume was 50 μl. To assess inhibition of the assay by 3TCTP, the same protocol was followed but with the addition of 0.8 pmol of 3TCTP to the reaction mixtures.
The reaction was initiated by the addition of the enzyme and continued for 30 min at 37°C. Duplicate aliquots from each reaction mixture were spotted onto DE81 paper circles presoaked with cold 5% trichloroacetic acid-1% sodium pyrophosphate solution (wt/vol). The paper circles bind oligonucleotides. After drying, the paper circles were washed three times (once with 8 ml and twice with 4 ml) for 5 min each time with cold 5% trichloroacetic acid-1% sodium pyrophosphate solution to remove unincorporated nucleotides and then rinsed twice with 95% ethanol (3 ml). The paper circles were dried and counted in Ultima Gold scintillant (4 ml) to assess the amount of product formed. Each experiment was performed in duplicate on three separate occasions.
For the preincubation procedure, the same reaction mixtures were prepared but no [3H]dCTP was added. The total mixture volume was 45 μl. The reaction was initiated by the addition of the enzyme for 30 min at 37°C. Following this period, [3H]dCTP (24 pmol) was added to each reaction mixture, and the reaction was reinitiated for 30 min at 37°C. After the second incubation period, the protocol was the same as that described for the single incubation. Each experiment was performed in duplicate on three separate occasions.
(c) Determination of standard 3TCTP inhibition curve.
The standard inhibition curve was determined using the template primer alone at a dilution of 1:400 and 3TCTP at 0, 0.05, 0.1, 0.2, 0.4, and 0.8 pmol. The preincubation procedure was used.
(d) Effect of PBMC extracts on the 3TCTP standard inhibition curve.
Before concentrations of 3TCTP in extracts from HIV-infected patients could be quantified, it was essential that any interference with the assay by cell extracts be identified and minimized. Standard 3TCTP inhibition curves were determined in the absence and presence of PBMC extracts from healthy volunteers (no 3TCTP present). The extracts contained 5 × 106 cells and, using a specific DNA polymerase assay (18), were shown to have undetectable dCTP levels. This directly assessed whether any interference was due to factors other than endogenous dCTP. Extracts (5 μl from a total volume of 100 μl) were added to the reaction mixtures.
(e) Effect of PBMC extracts on the 3TCTP standard inhibition curve following preincubation with CuSO4.
The presence of enzymes in the extracts may be responsible for the interference observed in other RT assays (ZDVTP and stavudine TP [d4TTP] assays). This has been overcome by the addition of CuSO4, which is an inhibitor of DNA and RNase enzymes (13). Standard 3TCTP inhibition curves were determined as described previously. Additional curves were performed in the presence of PBMC extract (5 μl from a total volume of 100 μl; the total volume contains 5 × 106 cells) and CuSO4. The protocol for these curves was modified as follows: the reaction mixtures contained KCl, Triton X-100, Tris-HCl, dithiothreitol, MgCl2, 3TCTP standard, and PBMC extract (at the same concentrations as before) with CuSO4 (200 μM). The reaction mixtures were left for 20 min at room temperature. EDTA and BSA (at the same concentrations as before) were then added to the reaction mixtures and left for a further 15 min at room temperature. Template primer and enzyme were added as before, and the mixtures were preincubated at 37°C for 30 min. Finally, [3H]dCTP was added, and the reaction mixtures were reincubated as described previously.
(f) Effect of dCTP on the 3TCTP standard inhibition curve.
Standard 3TCTP inhibition curves were performed as described above (c). Additional tubes contained a standard amount of 3TCTP (0.1 pmol) in the absence and presence of dCTP (0, 0.01, 0.02, 0.05, and 0.1 pmol), and the degrees of inhibition were compared. Additionally, standard 3TCTP inhibition curves were repeated (0, 0.05, 0.2, and 0.8 pmol only) in the absence and presence of dCTP (0.5 pmol).
(ii) CBVTP assay development. (a) Comparison of inhibition by CBVTP with and without a preincubation procedure.
For the single-incubation procedure, control product formation (i.e., incorporation of [3H]dGTP into the template primer in the absence of inhibitor) was determined using a reaction mixture the same as that described for the 3TCTP assay except that [3H]dGTP (15.625 pmol) and 5 μl of template primer [poly(rC) · poly(dG)12–18; 2.5 U ml−1] at a dilution of 1:5, 1:10, 1:20, or 1:40 were added instead of the corresponding 3TCTP assay reagents. To assess inhibition of the assay by CBVTP, the same protocol was followed but with the addition of 0.1 pmol of CBVTP to the reaction mixtures.
For the preincubation procedure, the same reaction mixtures were prepared but no [3H]dGTP was added. The reaction was initiated by the addition of the enzyme, and following this period, [3H]dGTP (15.625 pmol) was added and the reaction was reinitiated. Spotting, washing, and counting were performed as described for the 3TCTP assay. These incubation experiments were also performed in duplicate on three separate occasions.
(b) Determination of standard CBVTP inhibition curve.
The standard inhibition curve was determined using the template primer at a dilution of 1:10 with CBVTP at 0, 0.05, 0.1, 0.2, 0.4, and 0.6 pmol for the single-incubation procedure and 0, 0.02, 0.04, 0.06, 0.08, and 0.1 pmol using the preincubation procedure. The single-incubation procedure was adopted for subsequent studies.
(c) Effect of PBMC extracts on the CBVTP standard inhibition curve.
Standard CBVTP inhibition curves were determined in the absence and presence of PBMC extracts from healthy volunteers (no CBVTP present). The extracts contained 5 × 106 and 10 × 106 cells. Extracts (5 μl from a total volume of 100 μl) were added to the reaction mixtures.
(d) Effect of dGTP on the CBVTP standard inhibition curve.
Standard CBVTP inhibition curves were repeated (0, 0.05, 0.2, and 0.6 pmol only) in the absence and presence of dGTP (0.05, 0.1, and 0.5 pmol).
Variability and recovery determination for the 3TCTP and CBVTP assays and quantification of 3TCTP and CBVTP in PBMC extracts from HIV-infected patients.
The interassay variability of the 3TCTP assay was determined by quantifying a 0.1-pmol 3TCTP quality control (QC) standard on six separate occasions.
Intra-assay variability of the 3TCTP assay was determined by quantifying 3TCTP concentrations from the same PBMC extracts six times in the same assay. The PBMCs were isolated from blood taken from HIV-infected patients receiving 3TC (150 mg twice daily [b.i.d.]) and were extracted in methanol and perchloric acid.
Recovery of the assay was determined by quantifying the standard 3TCTP inhibition curve with previously prepared PBMC extracts in the presence and absence of a 0.1-pmol 3TCTP QC. The PBMCs were isolated from blood taken from healthy volunteers and were extracted in methanol and perchloric acid. QCs were added to the reaction mixtures. The ability to recover these QCs was calculated by subtracting the amount of 3TCTP present in the standard curve alone from the amount of 3TCTP obtained in the standard curve plus QC. 3TCTP QCs were prepared independently of the standard curves. Assay recovery was also investigated by spiking PBMCs from healthy volunteers with 3TCTP (2 pmol) and then extracting the samples with 3TCTP as previously described. 3TCTP levels in these extracts were then determined in the usual manner and compared to a 3TCTP standard of the same amount present in the extract (0.1 pmol of 3TCTP, as only 5 μl of extract was added to the reaction mixture from the 100-μl total).
3TCTP concentrations were quantified in PBMC extracts from four HIV-infected patients. All patients were receiving 3TC (150 mg b.i.d.) as part of their combination regimen for at least 6 months. The patients were admitted to the Royal Liverpool Hospital day ward, and blood (20 ml) was collected at 0, 1, 2, 4, 8, 12, 16, 24, and 36 h. The patients omitted 3TC at 12 and 24 h (hence the 36-h profile). PBMCs were isolated and extracted in methanol and perchloric acid. dCTP levels in the extracts were determined (18), and the 3TCTP inhibition curve was standardized by adding to the curve an amount of dCTP similar to that present in the extracts.
In assays where PBMC extracts were added to the reaction mixtures, only 5 μl from the total extract volume of 100 μl was used.
The interassay variability of the CBVTP assay was determined by quantifying CBVTP concentrations from the same PBMC extracts on six separate occasions. PBMCs were isolated from blood taken from HIV-infected patients receiving ABC (300 mg b.i.d.) and were extracted in methanol and perchloric acid. The interassay variability of the CBVTP assay was also determined by quantifying 0.05 and 0.3 pmol of CBVTP QC standards on eight separate occasions.
The intra-assay variability of the CBVTP assay was determined by quantifying CBVTP concentrations from the same PBMC extracts six times in the same assay. PBMCs were isolated from blood taken from HIV-infected patients receiving ABC (300 mg b.i.d.) and were extracted in methanol and perchloric acid.
Recovery of the assay was determined by quantifying CBVTP from previously prepared PBMC extracts alone and in the presence of 0.05 or 0.3 pmol of CBVTP QCs, using the same protocol as for the 3TCTP assay. The ability to recover these QCs was calculated by subtracting the amount of CBVTP present in the extract alone from the amount of CBVTP obtained in the extract plus QC. CBVTP QCs were prepared independently of the standard curves. Assay recovery was also investigated by spiking PBMCs from healthy volunteers with CBVTP (3 pmol) and then extracting the samples with CBVTP as previously described. CBVTP levels were then determined in these extracts in the usual manner and compared to a CBVTP standard of the same amount present in the extract (0.15 pmol of CBVTP, as only 5 μl of extract was added to the reaction mixture from the 100-μl total).
CBVTP concentrations were quantified in PBMC extracts from six HIV-infected patients. All patients were receiving ABC (300 mg b.i.d.) as part of their combination regimen for at least 6 months. The patients were admitted to the Royal Liverpool Hospital day ward, and blood (20 ml) was collected at 0, 1, 2, 4, 6, 8, 12, and 24 h. The patients omitted ABC at 12 h (hence the 24-h profile). PBMCs were isolated and extracted in methanol and perchloric acid. Concentrations of dGTP were also quantified in these samples using a specific DNA polymerase assay (18).
In assays where PBMC extracts were added to the reaction mixtures, only 5 μl from the total extract volume of 100 μl was used.
Approval for each of the studies was obtained from relevant local ethics committees. All patients enrolled gave informed written consent. None of the patients were involved in both studies.
RESULTS
3TCTP assay development.
The dC and rI homopolymers were purified by HPLC (>95% pure) prior to being annealed in our laboratories. Although the actual yield of template primer formed is unknown, repeated preparation of the template primer over time did not alter the resulting amount of control product formation. This suggests consistency in the yield of template primer formed over time.
As the concentration of template primer was diluted, the amount of control product formation decreased using the single-incubation procedure. For example, at a 1:400 template primer dilution, control product formation was reduced to 25.9% of undiluted levels (Table 1). The addition of 0.8 pmol of 3TCTP had no effect on control product formation at any of the dilutions of template primer studied (Table 1).
TABLE 1.
Effect of altering the concentration of template primer on control product formation and inhibition by 3TCTP with and without a preincubation procedurea
| Template primer dilution | Control product formation (dpm) | % Inhibition by 0.8 pmol of 3TCTP |
|---|---|---|
| No preincubation | ||
| Undiluted | 54,903 | 5.2 |
| 1:20 | 49,907 | 6.6 |
| 1:40 | 46,691 | 5.7 |
| 1:400 | 14,210 | 4.6 |
| With preincubation | ||
| Undiluted | 79,514 | 13.1 |
| 1:20 | 61,630 | 35.1 |
| 1:40 | 47,826 | 34.1 |
| 1:400 | 14,533 | 88.0 |
Data are expressed as mean values from three separate experiments.
Due to the inability of 3TCTP to sufficiently inhibit control product formation, a preincubation procedure was adopted to assess whether inhibition could be observed when 3TCTP was allowed to be incorporated into the template primer before the addition of [3H]dCTP. Table 1 demonstrates the ability of 0.8 pmol of 3TCTP to inhibit control product formation as the template primer is diluted, using the preincubation procedure. As observed with the single-incubation procedure, there was a steady decline in control product formation as the template primer was diluted (e.g., at a 1:40 dilution, control product formation was reduced to 60.2% of undiluted levels). However, there was a concurrent increase in the degree of inhibition of product formation by 0.8 pmol of 3TCTP as the template primer was diluted. For example, 0.8 pmol of 3TCTP inhibited product formation by 35.1 and 88.0% at 1:20 and 1:400 template primer dilutions, respectively.
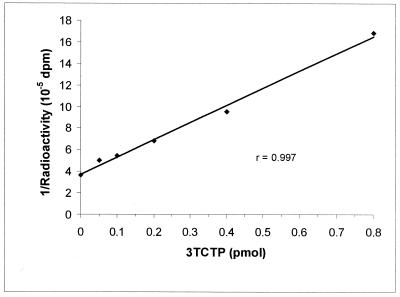
Standard 3TCTP inhibition curves were determined at 0, 0.05, 0.1, 0.2, 0.4, and 0.8 pmol of 3TCTP, using the preincubation procedure. Figure 1 shows a typical standard 3TCTP inhibition curve using this modified procedure. The coefficient of regression (r) values were usually greater than 0.99. The standard curves were also highly reproducible over time, suggesting that the 3TCTP standards were highly stable. Additionally, the stability of the stock 3TCTP solution was routinely checked by HPLC with UV detection.
FIG. 1.
Typical standard inhibition curve for the determination of 3TCTP. The assay conditions consisted of a preincubation procedure of 30 min at 37°C. The amount of [3H]dCTP was 24 pmol, with the template primer at a 1:400 dilution.
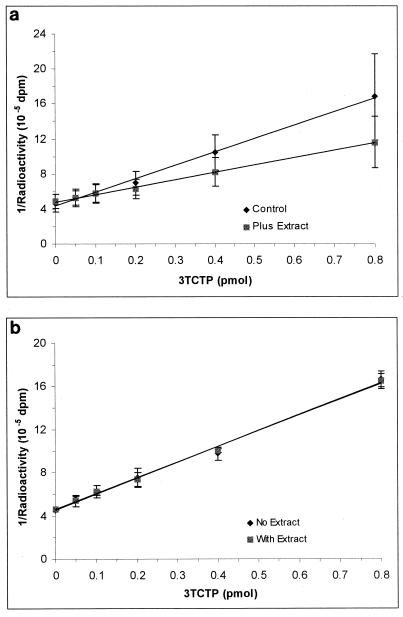
Figure 2a shows the effect of PBMC extracts (from 5 × 106 cells) on the standard 3TCTP inhibition curve. Interference was greatest at high concentrations of 3TCTP but was negated by the addition of CuSO4 (Fig. 2b).
FIG. 2.
Effect of PBMC extracts alone (a) and in the presence of CuSO4 (b) on the standard 3TCTP inhibition curve. The assay conditions consisted of three preincubation procedures of various lengths followed by a 30-min incubation at 37°C. The amount of [3H]dCTP was 24 pmol, with the template primer at a 1:400 dilution. The error bars indicate standard deviations of the mean.
The addition of dCTP (at any of the amounts investigated) to the 0.1-pmol 3TCTP standard had no effect on the degree of inhibition observed compared to that of the the 3TCTP standard alone (data not shown). Additional studies investigated the standard 3TCTP inhibition curve in the absence and presence of dCTP (0.5 pmol). The four-point standard 3TCTP inhibition curve was altered by 0.5 pmol of dCTP, with the product formation for each point of the curve being reduced by approximately 30% (data not shown). Therefore, later studies were corrected for high dCTP content by adding healthy-volunteer cell extract containing the same amount of dCTP to the 3TCTP standard inhibition curves.
CBVTP assay development.
As the concentration of template primer was diluted, the amount of control product formation decreased using the single-incubation procedure. For example, at a 1:40 template primer dilution, control product formation was reduced to 23.2% of 1:5 template primer dilution levels (Table 2). The addition of 0.1 pmol of CBVTP inhibited control product formation, and inhibition was greatest at a 1:40 template primer dilution (24.6% [Table 2]).
TABLE 2.
Effect of altering concentration of template primer on control product formation and inhibition by CBVTP with and without preincubation procedurea
| Template primer dilution | Control product formation (dpm) | % Inhibition by 0.1 pmol of CBVTP |
|---|---|---|
| No preincubation | ||
| 1:5 | 40,054 | 13.1 |
| 1:10 | 34,568 | 16.6 |
| 1:20 | 17,046 | 10.2 |
| 1:40 | 9,305 | 24.6 |
| With preincubation | ||
| 1:5 | 23,269 | 22.2 |
| 1:10 | 9,472 | 22.1 |
| 1:20 | 4,694 | 39.4 |
| 1:40 | 1,943 | 57.6 |
Data are expressed as mean values from three separate experiments.
A preincubation procedure was also adopted to assess whether the degree of inhibition by CBVTP observed using this procedure was different from that observed with the single-incubation procedure. The results are also shown in Table 2. As observed with the single-incubation procedure, there was a steady decline in control product formation as the template primer was diluted (e.g., at a 1:40 dilution, control product formation was reduced to 8.4% of 1:5 template primer dilution levels). There was a concurrent increase in the degree of inhibition of control product formation by 0.1 pmol of CBVTP as the template primer was diluted. For example, 0.1 pmol of CBVTP inhibited control product formation by 22.1 and 57.6% at 1:10 and 1:40 template primer dilutions, respectively. Overall, the degree of inhibition was greater in incubations using a preincubation procedure than when using a single-incubation procedure. It is also worth noting that the amount of control product formation was decreased (by approximately half) with a preincubation procedure compared to that with a single-incubation procedure.
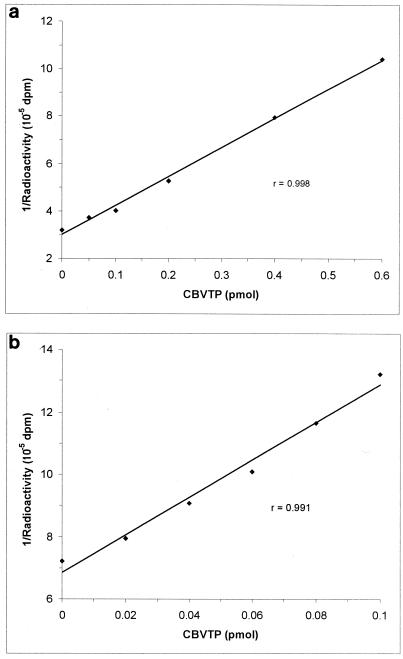
Standard CBVTP inhibition curves were determined at 0, 0.05, 0.1, 0.2, 0.4, and 0.6 pmol of CBVTP using the single-incubation procedure and at 0, 0.02, 0.04, 0.06, 0.08, and 0.1 pmol of CBVTP using the preincubation procedure. Figure 3a and b show typical standard CBVTP inhibition curves without and with the use of a preincubation procedure, respectively. The r values were usually greater than 0.99. The standard curves were also highly reproducible over time, suggesting that the CBVTP standards are highly stable. Additionally, the stability of the stock CBVTP solution was routinely checked by HPLC-UV.
FIG. 3.
Typical standard inhibition curves for the determination of CBVTP. The assay conditions consisted of either a single-incubation procedure of 30 min at 37°C (a) or a preincubation procedure of 30 min at 37°C (b). The amount of [3H]dGTP was 15.625 pmol, with the template primer at a 1:10 dilution.
PBMC extracts from blood samples obtained from three healthy volunteers were investigated for their ability to alter the standard CBVTP inhibition curve using a single-incubation procedure (Table 3). Extracts containing 5 × 106 and 10 × 106 cells were studied. There was no evidence of any interference either on control product formation itself or on the degree of inhibition by CBVTP.
TABLE 3.
Effect of PBMC cell extracts on CBVTP inhibition curve
| Amt of CBVTP (pmol) | Resultsa
|
||
|---|---|---|---|
| 0b | 5 × 106 | 10 × 106 | |
| 0 | 2.88 | 2.94 | 3.01 |
| 0.05 | 3.00 (4.1) | 3.16 (6.9) | 3.37 (10.7) |
| 0.1 | 3.27 (12.1) | 3.46 (15.0) | 3.58 (15.8) |
| 0.2 | 4.11 (30.0) | 4.13 (28.8) | 4.47 (32.6) |
| 0.4 | 5.55 (48.2) | 5.53 (46.8) | 5.60 (46.3) |
| 0.6 | 7.65 (62.4) | 8.07 (63.5) | 7.60 (60.4) |
Results are expressed as 1/counts (10−5 dpm) in the presence and absence of extract. The corresponding inhibition is shown in brackets. A single-incubation procedure was used in these experiments. The data are expressed as mean values from three separate experiments, each using cell extracts from different healthy volunteers.
Number of PBMC.
Additional studies investigated the standard CBVTP inhibition curve in the absence and presence of dGTP (0.05, 0.1, and 0.5 pmol), using the single-incubation procedure. The four-point standard CBVTP inhibition curve was unaffected by dGTP (data not shown).
Variability and recovery determination for the 3TCTP and CBVTP assays and quantification of 3TCTP and CBVTP in PBMC extracts from HIV-infected patients.
The limit of quantification of the 3TCTP assay varies depending on the number of cells present in the PBMC extracts. The greater the number of cells, the easier it is to quantify 3TCTP levels. The number of PBMCs isolated from blood varies considerably between patients and at different time points. Generally, >5 × 106 cells are required for the assay to be able to detect 3TCTP levels. However, from the standard curve itself (in the presence of healthy-volunteer cell extract), the limit of quantification was set at 0.05 pmol of 3TCTP. Therefore, for 5 × 106 cells, the limit of quantification of the 3TCTP assay would be 0.2 pmol/106 cells. The interassay variability of the 3TCTP assay determined from the 0.2-pmol 3TCTP QC analyzed on six separate occasions was calculated as ranging between 10 and 18%. The intra-assay variability of the 3TCTP assay determined by analyzing PBMC extracts six times in the same assay was calculated at 15%. Variability in the recovery of the 0.1-pmol QC in the presence of the standard 3TCTP inhibition curve plus PBMC extract was calculated as 16% (0.114 ± 0.017 pmol; n = 6) from the standard curve. Recovery of the 0.1-pmol 3TCTP when 2 pmol of 3TCTP was added to a healthy PBMC sample and then extracted was calculated as 90% (0.091 ± 0.012 pmol; n = 4) compared to the 0.1-pmol 3TCTP standard alone. Furthermore, repeated thawing and analysis of these 3TCTP-spiked PBMC extracts gave rise to consistent detection of these levels, suggesting a lack of degradation of 3TCTP (results not shown).
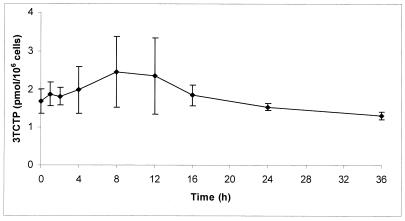
Figure 4 shows a 36-h pharmacokinetic profile of intracellular 3TCTP in PBMC extracts from four HIV-infected patients. 3TCTP concentrations were measurable throughout the 36 h of washout, ranging from 0.20 to 5.10 pmol/106 cells. There was considerable interpatient variability. Peak concentrations were observed between the 8- and 12-h time points. The levels of dCTP in these samples generally remained constant over the 36-h dosing interval and demonstrated a degree of interpatient variability similar to that observed for 3TCTP levels (data not shown).
FIG. 4.
Intracellular pharmacokinetic profile of 3TCTP in PBMC extracts from blood samples obtained from HIV-infected patients over 36 h of washout. The results shown are means ± standard errors of the mean from four patients.
The limit of quantification of the CBVTP assay varies depending on the number of cells present in the PBMC extracts. As with the 3TCTP assay, >5 × 106 cells are required for the assay to be able to detect CBVTP levels. However, from the standard curve itself, the limit of quantification was set at 0.02 pmol of CBVTP. Therefore, for 5 × 106 cells, the limit of quantification of the CBVTP assay would be 0.08 pmol/106 cells. The interassay variability of the CBVTP assay determined from the same PBMC extracts on six separate occasions was calculated as ranging between 5 and 15%. The interassay variability of the CBVTP assay determined from the 0.05-pmol CBVTP QC on eight separate occasions was calculated as ranging between 6 and 19%. The intra-assay variability of the CBVTP assay determined from analyzing PBMC extracts six times in the same assay was calculated at 11%. Variability in the recovery of QCs in the presence of PBMC extract was calculated as 9% for the 0.05-pmol QC (0.054 ± 0.005; n = 5) and 6% for the 0.3-pmol QC (0.309 ± 0.017; n = 12) from the standard curve. Recovery of the 0.15-pmol CBVTP when 3 pmol of CBVTP was added to a healthy PBMC sample and then extracted was calculated as 92% (0.138 ± 0.015 pmol; n = 4) compared to recovery with the 0.15-pmol CBVTP standard alone. Furthermore, repeated thawing and analysis of these CBVTP-spiked PBMC extracts gave rise to consistent detection of these levels, suggesting a lack of degradation of CBVTP (results not shown).
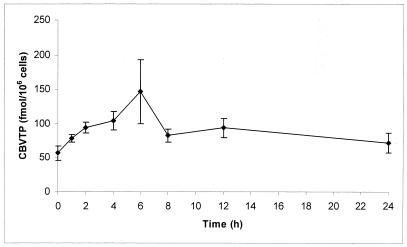
Figure 5 shows a 24-h pharmacokinetic profile of intracellular CBVTP in PBMC extracts from six HIV-infected patients. CBVTP concentrations were measurable throughout the 24 h of washout, ranging from 20 to 374 fmol/106 cells. There was considerable interpatient variability. Peak concentrations were observed at the 6-h time point. The levels of dGTP in these samples remained constant over the 24-h dosing interval (data not shown).
FIG. 5.
Intracellular pharmacokinetic profile of CBVTP in PBMC extracts from blood samples obtained from HIV-infected patients over 24 h of washout. The results shown are means ± standard errors of the mean from six patients.
DISCUSSION
These enzymatic assays were developed as viable alternatives to the HPLC-RIA procedure for the quantification of intracellular 3TCTP and CBVTP. The template primer used for the 3TCTP assay was an RNA-DNA template primer, which differs from the DNA template primers previously used in the determination of ZDVTP (4, 14) and CBVTP (as described above). However, the amount of product formed using this RNA-DNA template primer was considerable, suggesting its suitability for use in the assay. Likewise, the amount of product formed using the DNA template primer for CBVTP was also high.
Addition of standard amounts of 3TCTP to the reaction mixtures demonstrated that inhibition of [3H]dCTP incorporation could only be achieved at high levels of the inhibitor, using a single-incubation procedure. Subsequent studies investigated the extent of control product formation, and inhibition by 3TCTP, as the template primer was diluted. It was thought that the number of binding sites was very important to the amount of product formed. If there were too many sites (i.e., as was thought to be the case with the undiluted template primer), then [3H]dCTP would still become incorporated even though 3TCTP had already become incorporated into other strands of primer. Therefore, by restricting the number of binding sites through diluting the template primer, inhibition by 3TCTP might be observed. As the template primer was diluted, the reduction in available binding sites led to reduced product formation. However, no discernible inhibition of product formation by 0.8 pmol of 3TCTP was observed, even when the template primer was diluted.
Additional studies investigated the effect of reducing the amount of [3H]dCTP used in the reaction mixtures. Control product formation was reduced to unacceptable levels. At this stage, it was considered that, using the single-incubation procedure outlined, sufficient product formation and inhibition by 3TCTP were unlikely to be achieved. In contrast, the enzymatic assay for the determination of ZDVTP levels demonstrated that a similar procedure could be used without these problems. The differences can be explained by the relative efficiency of the two inhibitors for HIV RT in relation to their respective dNTPs. Previous workers demonstrated that ZDVTP is more efficient than (or at least as efficient as) dTTP as a substrate for HIV RT (5, 21, 22). In contrast, 3TCTP has been found to be only equally (or less) efficient as a substrate than dCTP for this enzyme (5, 7, 21). Therefore, it is perhaps not surprising that the 3TCTP assay needed to be modified. Further studies therefore investigated the effects of a preincubation procedure on both product formation and inhibition by 3TCTP. The preincubation step included the 3TCTP standards with no [3H]dCTP, thus allowing 3TCTP to become incorporated into the template primer first. Then [3H]dCTP was added, and the reaction mixtures were reincubated to allow incorporation of [3H]dCTP into the remaining available template primer strands. This procedure effectively prevented the occurrence of competition between 3TCTP and [3H]dCTP.
Initial studies using this modified procedure involved the effect of template primer dilution on control product formation and inhibition by 0.8 pmol of 3TCTP. As observed with the single-incubation experiments, as the amount of template primer was diluted there was a subsequent fall in control product formation. However, the presence of 0.8 pmol of 3TCTP resulted in inhibition of product formation, with a greater degree of inhibition noted as the template primer was diluted. Thus, the 1:400 template primer dilution was chosen for all future studies. These results demonstrated the viability of the modified procedure for determining inhibition by 3TCTP.
In contrast to the 3TCTP assay, the CBVTP assay did not suffer from these initial problems with regard to the single-incubation procedure. As observed for the 3TCTP assay, when the template primer was diluted, there was a concurrent decline in control product formation with the single-incubation procedure. These results again show that the amount of template primer is very important to the amount of product that can be formed, due to the number of binding sites present (i.e., diluting the template primer will restrict the incorporation of [3H]dGTP). The addition of 0.1 pmol of CBVTP resulted in inhibition of this control product formation, with the largest degree of inhibition observed when the template primer was at its most dilute. Similarly to ZDVTP, CBVTP is a much more efficient substrate than 3TCTP for HIV RT (6). Additional studies (results not shown) using a greater amount of [3H]dGTP (31.25 pmol) in the reaction mixture gave a reduction in the degree of inhibition of 0.1 pmol of CBVTP. This is probably due to the fact that competition will favor [3H]dGTP as it is increased in relation to CBVTP. A 1:10 template primer dilution was chosen for future studies, as product formation was sufficiently high while still allowing for a good degree of inhibition by CBVTP.
Although incorporation of [3H]dGTP was inhibited by CBVTP with the single-incubation procedure, the study was repeated to include a preincubation step in order to assess whether sensitivity could be improved. Diluting the template primer again led to a fall in the amount of control product formation, while the degree of inhibition was greater in incubations using the preincubation procedure than using the single-incubation procedure. These results are to be expected, as there is no initial competition between CBVTP and [3H]dGTP using the preincubation procedure, as CBVTP is allowed to become incorporated into the template primer before [3H]dGTP. Additional studies (results not shown) using a greater amount of [3H]dGTP (31.25 pmol) in the reaction mixture gave rise to no differences in the degree of inhibition by 0.1 pmol of CBVTP. This is because CBVTP had previously been allowed to become incorporated, so the amount of free template primer would still be the same. Therefore, although the amount of [3H]dGTP is increased, there will still only be the same number of sites available for incorporation.
The observed decrease in control product formation when using the preincubation procedure may involve a reduction in template primer stability as the length of the incubation is increased from 30 min to 1 h (overall).
Standard inhibition curves for assays with and without a preincubation step were reproducible and displayed a high recovery (results not shown). Although in the CBVTP assay inhibition was greater using the preincubation procedure, due to the reduction in template primer stability, the single-incubation procedure was adopted for routine use.
The final step was to determine intracellular 3TCTP and CBVTP in PBMC extracts from HIV-infected patients. However, before accurate quantification could be achieved, it was essential that any potential interference with the assays by cell extracts be identified and then minimized.
Initial experiments investigating potential interference with the 3TCTP assay used PBMC extracts from healthy volunteers on no antiretroviral therapy. Therefore, there was no 3TCTP present in the extracts. Secondly, using the dCTP DNA polymerase assay (18), these extracts were shown to have undetectable amounts of dCTP. The effect of these extracts on the standard 3TCTP inhibition curve was studied to assess whether any interference observed was due to factors other than the presence of endogenous dCTP, which would otherwise compete with both [3H]dCTP and 3TCTP for incorporation into the template primer. The results demonstrated that there is some interference caused by the PBMC extracts. Although the interference appears greatest with large amounts of 3TCTP, this is probably just a function of the reciprocal plot. The interference was not due to dCTP, as none was detected in these samples. Therefore, interference may be due to either the solvents that the extracts are in, active enzymes present in the extracts, or both.
Active enzymes have been thought responsible for interference observed with the ZDVTP and d4TTP assays (13) and so may be involved in the interference observed with the 3TCTP assay. The addition of CuSO4 reversed the inhibition observed with these extracts. CuSO4 is an inhibitor of DNA and RNase enzymes that are probably interfering with the assay. However, CuSO4 itself can also interfere with the assay by inhibiting the HIV RT enzyme (13). This led to further modification of the assay, where CuSO4 was first allowed to destroy the active enzymes present in the PBMC extracts and then BSA was added, which binds the CuSO4, thus inactivating it. Therefore, the use of CuSO4 minimizes interference observed with these types of extracts. But what about those containing endogenous dCTP? The addition of dCTP (up to 0.1 pmol) had no effect on the observed degree of inhibition by 3TCTP compared to 3TCTP standard alone (data not shown). This is probably due to the fact that in each reaction mixture there is 24 pmol of [3H]dCTP present, so the presence of small quantities of dCTP should have a negligible effect. However, when patient sample extracts are used with higher cell numbers, the levels of dCTP in these samples are often higher than 0.1 pmol. Additional studies demonstrated that larger amounts of dCTP (0.5 pmol) did affect product formation. This level of dCTP is higher than that detected in most patient samples. However, standardizing the inhibition curve through addition of dCTP itself, or healthy-volunteer extracts that contain the same amount of dCTP, can overcome this interference by higher dCTP levels.
In contrast, the standard CBVTP inhibition curve was not affected in the presence of healthy-volunteer PBMC extracts. This assay may not be susceptible to interference caused by active enzymes, as was observed with the 3TCTP assay. Furthermore, the addition of dGTP, even at levels that are not observed in PBMC extracts, had no effect on the standard CBVTP inhibition curve. This apparent lack of interference suggests that CBVTP levels in PBMC extracts from HIV-infected patients can be quantified without the need for any preincubations or correction of the standard inhibition curve.
The intracellular pharmacokinetic profiles of 3TCTP and CBVTP levels in PBMC extracts from HIV-infected patients were determined. For both 3TCTP and CBVTP, there was a large degree of variation between patients. However, these results can be expected due to the large amount of variation previously observed with other nucleoside analogues used to treat HIV infection (1, 2, 8, 11, 15, 16, 19, 20). Variation in these levels may involve a number of factors, including bioanalytical limitations; patient factors, such as immune status and cellular function; and pharmacokinetic or pharmacodynamic factors, such as endogenous enzyme activities and competition with endogenous nucleosides for these enzymes.
The intracellular levels of 3TCTP were measurable throughout the 36 h of washout, with the highest levels noted between the 8- and 12-h time points. This suggests a long half-life for 3TCTP in cells. Previous studies both in vivo and in vitro have observed an elimination half-life of 3TCTP of over 10 h (3, 10, 12, 17). The 3TC phosphate measurements by Moore et al. using combined HPLC-RIA were part of a collaborative project between Glaxo-SmithKline and our laboratory, with results indicating a median half-life of 3TCTP of over 15 h (12). Samples from four of the patients who took part in the study were then used to determine 3TCTP levels by our newly developed enzymatic assay for comparison. The results obtained (Fig. 4) show a pattern almost identical to those found previously using the combined HPLC-RIA procedure (12). In contrast, studies by Rodriguez et al. using an LC-MS-MS procedure suggested a median half-life of 3TCTP of approximately 32 h (17). These studies support the hypothesis that once-daily dosing may be possible. This is currently undergoing clinical trial.
The intracellular levels of CBVTP were also measurable throughout the 24 h of washout, with the highest levels noted at the 6-h time point. This also suggests a long half-life for CBVTP in cells. Since the Ki for inhibition by CBVTP of incorporation of dGTP into DNA by HIV type 1 RT has been reported to be 21 nmol/liter (6), these data point to an above-inhibitory level of CBVTP within the cell throughout the 24-h interval (50 fmol/106 cells = 100 nmol/liter). This suggests that once-daily dosing of ABC may also be possible, and this is also currently under investigation (M. Harris, S. Jutha, D. J. Back, S. Kewn, R. Marina, and J. S. G. Montaner, Abstr. 8th Conf. Retrovir. Opportun. Infect., abstr. P746, 2001).
The quantification of intracellular 3TCTP and CBVTP levels in PBMCs from HIV-infected patients is a viable option using these procedures and should prove to be of clinical use in the future. The assays require a lower number of cells in order to determine ddNTP levels (<10 × 106 cells). Therefore, less blood is collected from the patient. This is a very important advantage, as it is sometimes difficult to collect a large number of cells from HIV-infected patients, especially those at advanced stages of disease who may be cytopenic. While the assays are less time-consuming than combined HPLC-RIA methods, unlike the latter they cannot be used to determine mono- and diphosphates of ddNs. They also provide an inexpensive alternative to LC-MS-MS procedures, and due to the unique template primers used for each assay, they are more specific. The assays are also highly reproducible, displaying good inter- and intra-assay variability, in contrast to previous studies (14).
There are some drawbacks to using enzymatic assays to determine ddNTP levels. Interference by cell extracts was observed, specifically with the 3TCTP assay. However, this can be overcome to ensure an accurate determination of 3TCTP levels. Other drawbacks to the enzymatic assays are that specific assays are required to determine each ddNTP. In contrast, LC-MS-MS procedures can determine the levels of more than one type of ddNTP in the same assay (17). Additionally, ddNs from the same class (e.g., the two cytidine analogues 3TC and zalcitabine [ddC]) cannot be present in the same extract, as they will both compete for incorporation into the template primer. However, ddNs from the same class are rarely if ever coadministered. Interference resulting from other classes of ddNTP is unlikely due to the unique template primers used for each assay, which are specific for one class of ddNTP. Although ddNs are described as inhibitors of RT, they do not inhibit the function of the enzyme itself, only the elongation of the DNA chain by acting as chain terminators. This enables the assays to determine the levels of multiple classes of ddNTP in the same extract without any interference occurring. Furthermore, the RT in the reaction mixtures is present in excess, so other inhibitors of RT (e.g., nevirapine, a nonnucleoside RT inhibitor) do not interfere with the assay until they are at extremely high concentrations (data not shown).
LC-MS-MS procedures utilize an internal standard to allow for correction of day-to-day variability caused by changes in injection volumes and extraction prior to MS analysis (17). This helps to ensure an accurate determination of the metabolite(s) present in the cell extract. Any variability resulting from the extraction procedure described here cannot be corrected for by use of an internal standard, as the assays only measure one ddNTP. The assays also use exact volumes of extract, so an internal standard is not necessary. Moreover, the levels of 3TCTP in PBMCs quantified here in the same samples from HIV-infected patients compare very favorably to 3TCTP levels determined by Moore et al. (12). CBVTP levels determined by Glaxo-SmithKline using an LC-MS-MS procedure were mirrored by results from our CBVTP assay (unpublished data).
In conclusion, these assays represent a viable option for the quantification of intracellular 3TCTP and CBVTP levels.
Acknowledgments
We acknowledge financial support from Glaxo-SmithKline Research and Development, Greenford, United Kingdom.
REFERENCES
- 1.Barry, M. G., M. Wild, G. J. Veal, D. J. Back, A. M. Breckenridge, R. M. Fox, N. J. Beeching, F. J. Nye, P. Carey, and D. Timmins. 1994. Zidovudine phosphorylation in HIV-infected patients and seronegative volunteers. AIDS 8:F1–F5. [DOI] [PubMed] [Google Scholar]
- 2.Barry, M. G., S. H. Khoo, G. J. Veal, P. G. Hoggard, S. E. Gibbons, E. G. L. Wilkins, O. Williams, A. M. Breckenridge, and D. J. Back. 1996. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 10:1361–1367. [DOI] [PubMed] [Google Scholar]
- 3.Cammack, N., P. Rouse, C. L. P. Marr, P. J. Reid, R. E. Boehme, J. A. V. Coates, C. R. Penn, and J. M. Cameron. 1992. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem. Pharmacol. 43:2059–2064. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Y.-C., G. E. Dutschman, K. F. Bastow, M. G. Samgadharan, and R. Y. C. Ting. 1987. Human immunodeficiency virus reverse transcriptase: general properties and its interaction with nucleoside triphosphate analogs. J. Biol. Chem. 262:2187–2189. [PubMed] [Google Scholar]
- 5.Cherrington, J. M., M. D. Fuller, A. S. Mulato, S. J. W. Allen, S. C. Kunder, M. A. Ussery, Z. Lesnikowski, R. F. Schinazi., J.-P. Sommadossi, and M. S. Chen. 1996. Comparitive kinetic analyses of interaction of inhibitors with rauscher murine leukemia virus and human immunodeficiency virus reverse transcriptase. Antimicrob. Agents Chemother. 40:1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daluge, S. M., S. S. Good, M. B. Faletto, W. H. Miller, M. H. St. Clair, L. R. Boone, M. Tisdale, N. R. Parry, and J. E. Reardon. 1997. 1592U89, a novel carbocyclic nucleoside analog with potent selective anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 41:1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray, N. M., C. L. P. Marr, C. R. Penn, J. M. Cameron, and R. C. Bethell. 1995. The intracellular phosphorylation of (−)-2′-deoxy-3′-thiacytidine (3TC) and the incorporation of 3TC 5′-monophosphate into DNA by HIV-1 reverse transcriptase and human DNA polymerase γ. Biochem. Pharmacol. 50:1043–1051. [DOI] [PubMed] [Google Scholar]
- 8.Hoggard, P. G., J. Lloyd, S. H. Khoo, M. G. Barry, L. Dann, S. E. Gibbons, E. G. Wilkins, C. Loveday, and D. J. Back. 2001. Zidovudine phosphorylation determined sequentially over 12 months in human immunodeficiency virus-infected patients with or without previous exposure to antiretroviral agents. Antimicrob. Agents Chemother. 45:976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kewn, S., G. J. Veal, P. G. Hoggard, M. G. Barry, and D. J. Back. 1997. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem. Pharmacol. 54:589–595. [DOI] [PubMed] [Google Scholar]
- 10.Kewn, S. 1999. The biochemical pharmacology of the anti-HIV nucleoside analogues. Ph.D. thesis. University of Liverpool, Liverpool, England.
- 11.Moore, J. D., G. Valette, A. Darque, X. J. Zhoo, and J.-P. Sommadossi. 2000. Simultaneous quantitation of the 5′-triphosphate metabolites of zidovudine, lamivudine, and stavudine in peripheral mononuclear blood cells of HIV infected patients by high-performance liquid chromatography tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 11:1134–1143. [DOI] [PubMed] [Google Scholar]
- 12.Moore, K. H., J. E. Barrett, S. Shaw, G. E. Pakes, R. Churchus, A. Kapoor, J. Lloyd, M. G. Barry, and D. J. Back. 1999. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS 13:2239–2250. [DOI] [PubMed] [Google Scholar]
- 13.Phiboonbanakit, D. 1998. The interrelationship between intracellular thymidine and thymidine analogues phosphorylation. Ph.D. thesis. University of Liverpool, Liverpool, England.
- 14.Robbins, B. L., J. Rodman, C. McDonald, R. V. Srinivas, P. M. Flynn, and A. Fridland. 1994. Enzymatic assay for measurement of zidovudine triphosphate in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 38:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins, B. L., T. T. Tran, F. H. Pinkerton, F. Akeb, R. Guedj, J. Grassi, D. Lancaster, and A. Fridland. 1998. Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 42:2656–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodman, J. H., B. L. Robbins, P. M. Flynn, and A. Fridland. 1996. A systemic and cellular model for zidovudine plasma concentrations and intracellular phosphorylation in patients. J. Infect. Dis. 174:490–499. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez, J. F., J. L. Rodriguez, J. Santana, H. Garcia, and O. Rosario. 2000. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 44:3097–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman, P. A., and J. A. Fyfe. 1989. Enzymatic assay for deoxynucleoside triphosphates using synthetic oligonucleotides as template primers. Anal. Biochem. 180:222–226. [DOI] [PubMed] [Google Scholar]
- 19.Slusher, J. T., S. K. Kuwahara, F. M. Hamzeh, L. D. Lewis, D. M. Kornhauser, and P. S. Lietman. 1992. Intracellular zidovudine (ZDV) and ZDV phosphates as measured by a validated combined high-pressure liquid chromatography-radioimmunoassay procedure. Antimicrob. Agents Chemother. 36:2473–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stretcher, B. N., A. J. Pesce, B. A. Geisler, and W. H. Vine. 1991. A coupled HPLC/radioimmunoassay for analysis of zidovudine metabolites in mononuclear cells. J. Liq. Chromatogr. 14:2261–2272. [Google Scholar]
- 21.Ueno, T., and H. Mitsuya. 1997. Comparative enzymatic study of HIV-1 reverse transcriptase resistant to 2′,3′-dideoxynucleotide analogues using the single-nucleotide incorporation assay. Biochemistry 36:1092–1099. [DOI] [PubMed] [Google Scholar]
- 22.Ueno, T., T. Shirasaka, and H. Mitsuya. 1995. Enzymatic characterization of human immunodeficiency virus type 1 reverse transcriptase resistant to multiple 2′,3′-dideoxynucleoside-5′-triphosphates. J. Biol. Chem. 270:23605–23611. [DOI] [PubMed] [Google Scholar]