Abstract
Ceftriaxone is highly effective clinically in patients with Lyme disease. We studied a representative invasive human isolate of Borrelia burgdorferi for which the MBC of ceftriaxone was 0.050 μg/ml. A once-per-day dosage regimen of ceftriaxone (50 mg/kg/dose) administered intramuscularly for 5 days was 100% effective in sterilizing tissue samples of C3H mice infected with this strain of B. burgdorferi, regardless of whether the mice were being treated concomitantly with a corticosteroid. Administration of the same five doses of ceftriaxone at 6-h intervals over just 24 h was also 100% effective. These experiments suggest that shorter courses of antibiotics than those currently recommended should be considered for study in patients with early uncomplicated Lyme disease.
Lyme disease caused by the spirochete Borrelia burgdorferi is the most common vector-borne infection in North America. In vitro, B. burgdorferi is susceptible to the lethal action of several different antibiotic classes. For example, the MBC of the expanded-spectrum cephalosporin ceftriaxone is usually ≤0.4 μg/ml and this agent is also effective clinically (2, 6). While most authoritative sources recommend treatment regimens for patients with Lyme disease similar to that employed for other spirochetal diseases, duration of therapy is nonetheless a topic of controversy (20, 21). The treatment durations recommended in the Infectious Diseases Society of America Practice Guidelines on the Treatment of Lyme Disease range from 14 to 28 days, which is considerably longer than those used successfully to treat patients with infection due to relapsing-fever borrelia (one dose to 10 days of therapy) (4, 19).
Unlike most other spirochetal microorganisms, B. burgdorferi can be readily cultured in vitro and a variety of rodents and other laboratory animals are suitable for experimental studies. In animal experiments, 5 to 10 days of treatment of rodents (7–9) and 30 days of therapy of dogs (14) using the appropriate dosage and type of antibiotic cure the infection on the basis of the inability to recover B. burgdorferi by culture of postmortem tissue samples. Shorter courses of treatment, however, have not been systematically studied.
The purpose of this study was to determine if ceftriaxone therapy of mice could be shortened to a time period estimated to achieve a drug level in serum in excess of the MBC for B. burgdorferi for a time period shorter than that which would follow a single parenteral dose given to humans. The second objective was to determine if immunosuppressive doses of corticosteroids affect the activity of short-course therapy with ceftriaxone in vivo.
MATERIALS AND METHODS
Animals.
Female C3H mice (4 to 5 weeks old) were obtained from Charles River Laboratories (Wilmington, Mass.). All mice were housed in a filtered-air environment maintained at 20 ± 2°C. Antibiotic-treated and non-antibiotic-treated (control) mice were kept in separate cages for the infectivity experiments.
Bacteria and bacterial cultures.
A strain of B. burgdorferi designated BL206 was used for these experiments. BL206 was isolated from the blood of a patient who presented with erythema migrans. This organism was maintained by passaging in Barbour-Stoenner-Kelly (BSK) medium and used for the infectivity experiments (see below) after appropriate dilutions were made in BSK medium. The organism had been passaged between five and eight times.
The MIC and MBC of ceftriaxone for BL 206 were determined by a modified microplate dilution assay (13). Triplicate wells contained 5 × 105 B. burgdorferi bacteria in BSK medium with and without diluted ceftriaxone (Roche, Nutley, N.J.). After incubation for 48 h, wells were examined by dark-field microscopy and surviving Borrelia bacteria were enumerated as previously described (12).
Induction of immunosuppression.
At intervals designated to coincide with antibiotic treatment (described below), one group of mice was given cortisone acetate (Sigma, St. Louis, Mo.) once daily at a dose of 100 mg/kg of body weight for 5 days. The cortisone acetate was suspended and diluted in sterile saline (Abbott Laboratories, North Chicago, Ill.) and injected subcutaneously in a volume of 0.1 ml. The dosage and treatment regimens used here were similar to those used previously by others (3, 22) to immunocompromise mice during experimental Chlamydia or Legionella infection.
Infectivity experiments.
Mice infected with BL206 were treated with (i) ceftriaxone (Roche) over a 5-day period, (ii) ceftriaxone over a 24-h period, or (iii) both cortisone acetate and ceftriaxone for 5 days. Control animals included mice infected with BL 206 that were treated with only sterile saline or cortisone acetate.
Mice were infected by injection with a tuberculin syringe intradermally in the abdominal area with 100,000 B. burgdorferi bacteria in a volume of 0.1 ml of BSK medium. At 14 to 21 days later, the mice were given a single daily dose of either saline, ceftriaxone (50 mg/kg/dose), cortisone acetate (100 mg/kg/dose), or ceftriaxone (50 mg/kg/dose) plus cortisone acetate (100 mg/kg/dose) for 5 days. The saline and ceftriaxone injections were given in a volume of 0.1 ml by the intramuscular route. In a separate experiment, one group of infected mice received the five doses of ceftriaxone (50 mg/kg/dose) over a 24-h period, with each dose given once every 6 h (i.e., at 0, 6, 12, 18, and 24 h). Treatment was initiated at a time point at which infection with BL 206 is known to be widely disseminated in C3H mice on the basis of the results of our prior investigations (17).
Five to 7 days after completion of the saline, ceftriaxone, or cortisone acetate treatment, each mouse was humanely euthanatized and exsanguinated and urinary bladder and ear tissue samples (about 7 by 10 mm) were taken to ascertain the infection status of each of the infected and treated mice (13). Separate extracts of excised urinary bladders and ears (collected aseptically) were prepared by mincing the tissues finely with scissors and forceps and suspending them in a small volume (0.3 to 0.4 ml) of BSK medium. After the heavy tissue particles settled out, each of the extract suspensions was added to separate snap-cap tubes containing 4 ml of BSK medium. Tubes were incubated at 33°C, and the cultures were examined microscopically (by dark-field or phase-contrast microscopy) at 2- and 4-week intervals for the presence of live, motile spirochetes.
Ceftriaxone measurements in mouse plasma and pharmacokinetic analysis.
Separate groups of age- and sex-matched C3H mice were evaluated for levels of ceftriaxone in plasma at various times after administration of the 24-h ceftriaxone treatment regimen. Whole blood was collected via the ventral tail vein or by cardiac puncture using a tuberculin syringe and placed into heparinized microcentrifuge tubes at 1, 2, 3, 4, 5, 6, and 7 h after administration of the last of the five intramuscular injections of ceftriaxone. About 0.5 to 0.7 ml of plasma per mouse was transferred to a screw-cap 2.0-ml Micro tube (Sarstedt, Newton, N.C.) after centrifugation of the whole blood at 8,000 × g for 5 min. The nonpooled plasma samples were frozen immediately at −70°C until tested. At least three plasma samples were collected at each time point. Ceftriaxone levels were determined by a high-performance liquid chromatographic procedure (1) done by a commercial laboratory (Department of Laboratories, Barnes-Jewish Hospital, St. Louis, Mo.). The lower limit of detection by the assay was 0.2 μg/ml, and the upper limit was 200 μg/ml. Plasma samples were shipped frozen to the testing laboratory by overnight delivery. The data were analyzed by using an open two-compartment pharmacokinetic model (PK Analyst; Micro Math Scientific Software, Salt Lake City, Utah).
RESULTS
According to a microplate dilution assay, the MIC and MBC of ceftriaxone for the B. burgdorferi BL 206 isolate studied were 0.025 and 0.050 μg/ml, respectively. The first set of in vivo experiments was designed to determine whether a 5-day course of ceftriaxone is efficacious in mice with early-stage disseminated B. burgdorferi infection and whether coadministration of cortisone acetate would interfere with the antimicrobial effects of ceftriaxone therapy. As shown in Table 1, 5 days of therapy with ceftriaxone was highly effective in eliminating borrelial infection due to the invasive human isolate BL 206 and was significantly more effective than saline treatment (P < 0.001 [Fisher’s exact test, two tailed]). Concomitant administration of cortisone acetate did not impair antibiotic efficacy. Live spirochetes could not be recovered from the urinary bladders or ears of any of the steroid-treated mice that received ceftriaxone.
TABLE 1.
Efficacy of ceftriaxone in B. burgdorferi-infected mice treated with and without cortisone acetate
| No. of infected mice | Postinfection treatment | No. of mice with positive cultures for B. burgdorferi in:
|
|
|---|---|---|---|
| Urinary bladder | Ear | ||
| 10 | Saline | 10 | 10 |
| 6 | Ceftriaxone | 0 | 0 |
| 7 | Ceftriaxone + CAa | 0 | 0 |
| 3 | CA | 3 | 3 |
CA, cortisone acetate.
The second set of experiments was designed to determine whether short-term treatment with ceftriaxone (five doses administered over a 24-h period) is as effective as the above-described 5-day treatment regimen in eradicating viable B. burgdorferi in mice at an early stage of infection. As shown in Table 2, ceftriaxone administered over 24 h was significantly more effective than saline in eliminating viable B. burgdorferi (P = 0.002 [Fisher’s exact test, two tailed]). There was no difference in efficacy between the 24-h and 5-day treatment courses; 100% of the evaluable mice in both groups had negative bladder and ear tissue cultures.
TABLE 2.
Efficacy of five doses of ceftriaxone given over either 24 h or 5 days to C3H mice infected with B. burgdorferi
| No. of infected mice | Treatment regimen | No. of mice with positive cultures for B. burgdorferi in:
|
|
|---|---|---|---|
| Urinary bladder | Ear | ||
| 6 | Saline | 6 | 5a |
| 5b | 24-h course of ceftriaxone | 0 | 0 |
| 5 | 5-day course of ceftriaxone | 0 | 0 |
Among the saline-treated mice, one ear culture from one mouse was unevaluable due to contamination with other bacteria.
Of the original six mice in this group, one was deemed unevaluable because the ear culture was contaminated with other bacteria.
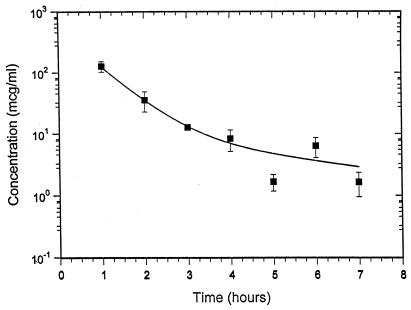
Ceftriaxone was detectable in plasma at all of the time points studied, and the levels are plotted in Fig. 1. The data were best fitted by a two-compartment model. A least-squares analysis was carried out with a total of 26 datum points; the mean ceftriaxone level in plasma and the standard error for each time point are presented. The half-time of elimination for the first phase of distribution and elimination (t1/2α) was 29 min. The terminal elimination half-time (t1/2β) was 200 min.
FIG. 1.
Ceftriaxone levels in the plasma of C3H mice at various time points after administration of the fifth and last dose of ceftriaxone (50 mg/kg given intramuscularly every 6 h). The mean ceftriaxone level and the standard error are plotted for each time point.
DISCUSSION
This study has demonstrated that five doses of ceftriaxone given over either 24 h or 5 days cures B. burgdorferi infection in C3H mice, as defined by the absence of positive cultures in two key tissue sites 5 to 7 days after completion of therapy. Five days of therapy was effective regardless of whether the mice were immunosuppressed with corticosteroids. Unlike most prior studies on the effects of antibiotics on laboratory animals, our study involved an invasive human strain of B. burgdorferi initially recovered from the blood of a patient with erythema migrans. The MIC (0.025 μg/ml) and MBC (0.050 μg/ml) of ceftriaxone for this isolate, however, are similar to those reported for other strains of B. burgdorferi (10).
The lack of a detrimental effect of corticosteroid treatment on the success of a 5-day course of therapy was consistent with the findings reported by Kazragis et al. (7), who showed that a 9-day course of ceftriaxone reliably eradicated B. burgdorferi infection in mice with severe combined immunodeficiency (SCID mice).
The every-6-h dosage regimen was evaluated because the t1/2 of ceftriaxone in mice was believed to be ≤1 h on the basis of several reports (5, 11, 15, 16). Our data, however, suggest that the pharmacokinetics of ceftriaxone in C3H mice may be more complicated than previously believed and are consistent with a two-compartment model in which the t1/2 of the first phase is short (29 min) but in which there is a more prolonged terminal t1/2 of approximately 200 min. Some caution may be warranted in the interpretation of these results since, despite the relatively good fit of the data by least-squares analysis, certain of the individual-animal datum points are close to the sensitivity limit of the analytical method used. Supporting evidence for two-compartment kinetics exists for mice treated with other cephalosporins. In a study by van Ogtrop et al. (15) in which drug concentrations in plasma were measured for a 4-h period after dosing, cefoperazone, ceftazidime, and cefepime showed evidence of two-compartment elimination. Ceftriaxone, also evaluated in the Ogtrop study (15), did not show evidence of two-compartment elimination over the 4-h period, but our data demonstrate that the slower elimination phase could be missed unless datum points are extended beyond 4 h. On the basis of our analysis, administration of five doses of ceftriaxone over a 24-h period should result in levels of ceftriaxone in mouse plasma in excess of the MBC for the B. burgdorferi strain used in this study for just under 60 h, which is a shorter time period than would be expected for intramuscular administration of a single 125-mg dose of ceftriaxone to humans (data not shown). This is attributable to the approximately 7-h t1/2 of ceftriaxone in adult humans (11).
A limitation of this study is that only a single strain of B. burgdorferi was studied and therefore, before generalizing conclusions can be drawn, additional strains should be investigated. In addition, only a single class of antibiotic and only mice with recent B. burgdorferi infection were studied. In prior reports, however, a 5-day course of ceftriaxone eradicated B. burgdorferi infection in C3H mice independently of when the infection occurred and for as long as 90 days after the onset of infection (8).
Extrapolation from studies with laboratory animals to humans should be done with caution. However, it is interesting that Weber et al. (18) successfully treated a group of German patients with erythema migrans with single daily 1-g doses of ceftriaxone administered for just 5 days. Rigorous clinical trials are needed to determine more precisely the appropriate duration of antibiotic therapy for patients with early uncomplicated Lyme disease.
Acknowledgments
We thank William Golde, Felipe Cabello, and Jen Wei Chiao for advice and Eleanor Bramesco and Lisa Giarratano for assistance.
REFERENCES
- 1.Ascalone, V., and L. Dal Bo. 1983. Determination of ceftriaxone, a novel cephalosporin, in plasma, urine and saliva by high-performance liquid chromatography on an NH2 bonded phase column. J. Chromatogr. 272:357–366. [DOI] [PubMed] [Google Scholar]
- 2.Baradaran-Dilmaghani, R., and G. Stanek. 1996. In vitro susceptibility of thirty Borrelia strains from various sources against eight antimicrobial chemotherapeutics. Infection 24:60–63. [DOI] [PubMed] [Google Scholar]
- 3.Brieland, J. K., D. Loebenberg, F. Menzel, and R. S. Hare. 2000. Efficacy of SCH27899 in an animal model of Legionnaires’ disease using immunocompromised A/J mice. Antimicrob. Agents Chemother. 44:1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, T., P. K. Jones, and C. K. Wallace. 1978. Borrelia recurrentis infection: single-dose antibiotic regimens and management of Jarisch-Herxheimer reaction. J. Infect. Dis. 177:573–577. [DOI] [PubMed] [Google Scholar]
- 5.Gombert, M. E., L. B. Berkowitz, T. M. Aulicino, L. and DuBouchet. 1990. Therapy of pulmonary nocardiosis in immunocompromised mice. Antimicrob. Agents Chemother. 34:1766–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, R. C., C. Kodner, and M. Russell. 1987. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob. Agents Chemother. 31:164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazragis, R. J., L. L. Dever, J. H. Jorgensen, and A. G. Barbour. 1996. In vivo activities of ceftriaxone and vancomycin against Borrelia spp. in the mouse brain and other sites. Antimicrob. Agents Chemother. 40:2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody, K. D., R. L. Adams, and S. W. Barthold. 1994. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob. Agents Chemother. 38:1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mursic, V. P., B. Wilske, G. Schierz, M. Holmburger, and E. Sub. 1987. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur. J. Clin. Microbiol. 6:424–426. [DOI] [PubMed] [Google Scholar]
- 10.Nowakowski, J., D. McKenna, R. B. Nadelman, et al. 2000. Failure of treatment with cephalexin for Lyme disease. Arch. Fam. Med. 9:563–567. [DOI] [PubMed] [Google Scholar]
- 11.Patel, I. H., and S. A. Kaplan. 1984. Pharmacokinetic profile of ceftriaxone in man. Am. J. Med. 77:17–25. [PubMed] [Google Scholar]
- 12.Pavia, C. S., G. P. Wormser, and G. L. Norman. 1997. Activity of sera from patients with Lyme disease against Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S25–S30. [DOI] [PubMed] [Google Scholar]
- 13.Pavia, C. S., G. P. Wormser, J. Nowakowski, and A. Cacciapuoti. 2001. Efficacy of an everinomycin (SCH 27899) in vitro and in an animal model of Lyme disease. Antimicrob. Agents Chemother. 45:936–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straubinger, R. K., A. F. Straubinger, B. A. Summers, and R. H. Jacobson. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and effects of corticosteroids: an experimental study. J. Infect. Dis. 181:1069–1081. [DOI] [PubMed] [Google Scholar]
- 15.Van Ogtrop, M. L., H. Mattie, H. F. L. Guiot, E. Van Strijen, A.-M. Hazekamp-Van Dukkum, and R. Van Furth. 1990. Comparative study of the effects of four cephalosporins against Escherichia coli in vitro and in vivo. Antimicrob. Agents Chemother. 34:1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang, E., M. Simard, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2000. In vivo activity and pharmacokinetics of ziracin (SCH 27899), a new long-acting everinomycin antibiotic, in a murine model of penicillin-susceptible or penicillin-resistant pneumococcal pneumonia. Antimicrob. Agents Chemother. 44:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, G., C. Ojaimi, R. Iyer, et al. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber, K., V. Preac-Mursic, B. Wilske, R. Thurmayr, U. Neubert, and C. Scherwitz. 1990. A randomized trial of ceftriaxone versus oral penicillin for the treatment of early European Lyme borreliosis. Infection 18:91–96. [DOI] [PubMed] [Google Scholar]
- 19.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, et al. 200. Practice guidelines for the treatment of Lyme disease. Clin. Infect Dis. 31(Suppl. 1):S1–S14. [DOI] [PubMed] [Google Scholar]
- 20.Wormser, G. P. 1995. Lyme disease: insights into the use of antimicrobials for prevention and treatment in the context of experience with other spirochetal infections. Mt. Sinai J. Med. 62:188–195. [PubMed] [Google Scholar]
- 21.Wormser, G. P. 1995. Duration of therapy for Lyme borreliosis. J. Infect. Dis. 171:1379.. [DOI] [PubMed] [Google Scholar]
- 22.Yang, Y. S., C. C. Kuo, and W. J. Chen. 1983. Reactivation of Chlamydia trachomatis lung infection in mice by cortisone. Infect. Immun. 39:655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]