Abstract
Microsporidia are eukaryotic obligate intracellular protists that are emerging pathogens in immunocompromised hosts, such as patients with AIDS or patients who have undergone organ transplantation. We have demonstrated in vitro and in vivo that synthetic polyamine analogs are effective antimicrosporidial agents with a broad therapeutic window. CD8-knockout mice or nude mice infected with the microsporidian Encephalitozoon cuniculi were cured when they were treated with four different novel polyamine analogs at doses ranging from 1.25 to 5 mg/kg of body weight/day for a total of 10 days. Cured animals demonstrated no evidence of parasitemia by either PCR or histologic staining of tissues 30 days after untreated control animals died.
The nontaxonomic term “microsporidia” is used to refer to a group of obligate, intracellular spore-forming parasitic protists that belong to the phylum Microspora, which consists of 144 genera and over 1,000 species (34, 44). These organisms are ubiquitous in nature, with infections being described in both invertebrate and vertebrate hosts, including insects, fish, and mammals (34, 44). They have importance as agricultural pathogens and are emerging pathogens of humans. The first identified microsporidia was Nosema bombycis, the etiologic agent of prebrine in silkworms, and the first reported human microsporidian infection was in 1959 (25). The genera Nosema, Vittaforma, Brachiola, Pleistophora, Encephalitozoon, Enterocytozoon, Septata (reclassified to Encephalitozoon), and Trachipleistophora have been found in human infections. Of the microsporidia implicated in human infections, the most common are Enterocytozoon bieneusi and the Encephalitozoonidae: Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis (22). The most common clinical manifestation of microsporidiosis is infection of the digestive tract; but disseminated infection and infections of the ocular, reproductive, respiratory, muscle, excretory, and nervous systems also occur (30, 41, 43). The environmental sources of the microsporidia that infect humans are poorly characterized, but many of the pathogenic microsporidia have been demonstrated in water supplies. Encephalitozoonidae are widely distributed parasites of mammals and birds, and the onset of microsporidiosis has been associated with exposure to livestock, fowl, and pets (9).
It is likely that microsporidiosis is a common infection but is self-limited or asymptomatic in healthy hosts. In recent studies, microsporidia have been identified in up to 20% of children with diarrhea in underdeveloped countries (18, 38). Although initially regarded as rare, microsporidia are now well-recognized pathogens, especially in immunocompromised patients with human immunodeficiency virus infection. The reported prevalence rates for microsporidiosis vary between 2 and 70%, depending on the population studied and the diagnostic technique used. As is true for many opportunistic pathogens, highly active antiretroviral treatment has resulted in a decrease in the prevalence of these infections in individuals with human immunodeficiency virus infection. Asymptomatic carriage of microsporidia has been demonstrated in immunocompetent and immunocompromised patients. Coinfection with different microsporidian or other enteric pathogens can occur.
Two groups of drugs have been used in the treatment of microsporidiosis. The first class of com2pounds is the tubulin-binding benzimidazoles. The antihelmintic albendazole has been the benzimidazole of choice in the treatment of microsporidiosis; however, it has proved ineffective against E. bieneusi (11, 13, 20, 42). The second class of compounds comprises the antibiotic fumagillin and its derivatives. Fumagillin has shown efficacy in the treatment of E. bieneusi infections in AIDS patients, but its use was associated with thrombocytopenia (26). Additional therapeutic targets are needed for the treatment of microsporidian infections.
Recent advances in antitumor chemotherapy have taken advantage of the central role that the polyamines play in cell growth and differentiation (14, 24). These small molecules, commonly known as putrescine, spermidine, and spermine (Fig. 1), originate in living cells from the ornithine decarboxylase-mediated decarboxylation of ornithine. Putrescine, thus produced, is sequentially aminopropylated at its amino groups to produce spermidine and spermine in reactions mediated by spermidine and spermine synthases, in which decarboxylated S-adenosylmethionine is used as the aminopropyl donor. A salvage pathway exists whereby spermine and spermidine are N-acetylated in reactions mediated by spermidine-spermine acetyltransferase, with the N-acetyl derivatives then oxidatively cleaved with release of 3-acetamidopropionaldehyde. The backconversion pathway leads from spermine to spermidine and from spermidine to putrescine (24). We have recently shown that E. cuniculi has fully functional polyamine metabolic pathways, including synthesis and backconversion (1). The enzymes of the latter pathway are active in the preemergent spore stages of this member of the microsporidia, leading to active uptake of spermine and its catabolism to spermidine and putrescine (1).
FIG. 1.
Structures of natural polyamines.
Many polyamine analogs have been synthesized which interfere with polyamine functions and metabolism and which are transported into cells by the polyamine transport system. The therapeutic effects of select polyamine analogs are not blocked by the presence of exogenous natural polyamines (14, 23). As such, we considered that an attractive approach with a rational biochemical basis for the development of new antimicrosporidial drugs would be through the use of polyamine analogs that interfere with polyamine function and that are actively concentrated in microsporidia by polyamine transporters.
MATERIALS AND METHODS
Synthesis of polyamine analogues.
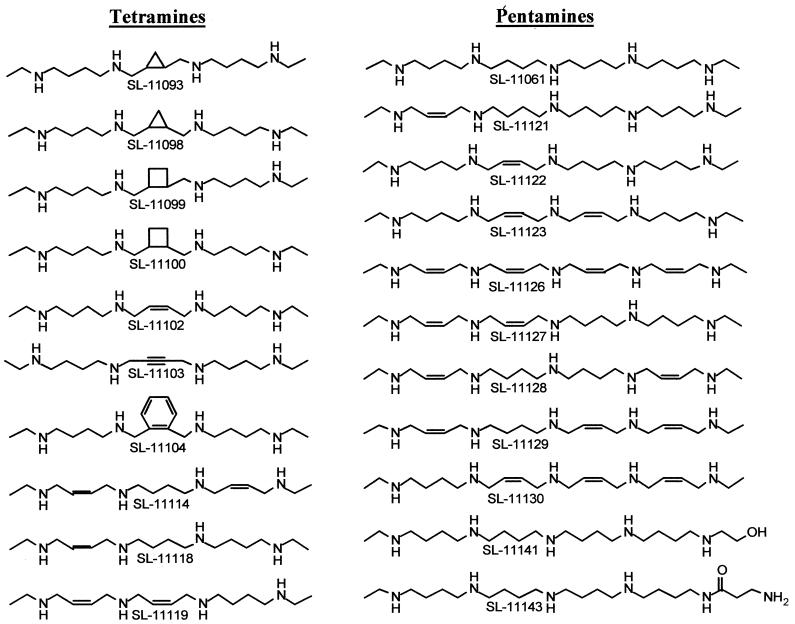
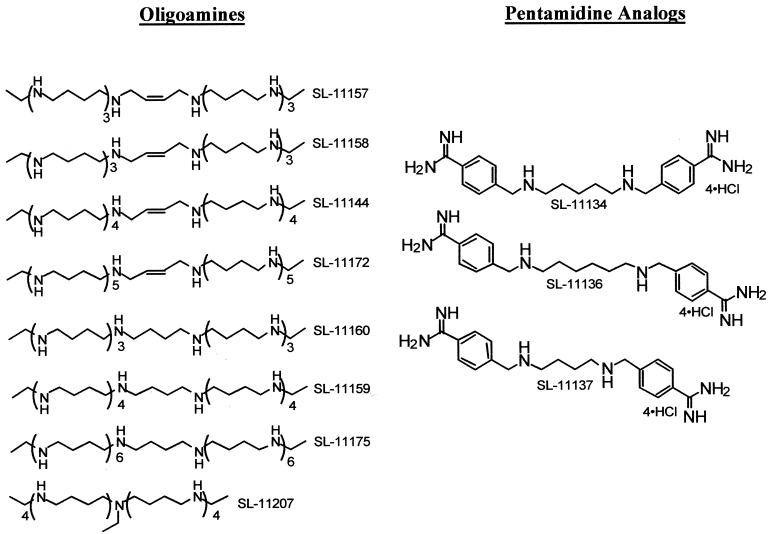
The syntheses of the tetramines and pentamines shown in Fig. 2 were recently reported (31, 37). The syntheses of the oligoamines shown in Fig. 3 were carried out by a general procedure. 1N-Monoethyl tetramides, pentamides, hexamides, and heptamides in which the amino groups are protected by mesitylenesulfonyl residues (prepared as described elsewhere [31, 37]) were dimerized by reaction with (E)- or (Z)-2-butene-1,4-diyl-bis(mesitylenesulfonate) to give the corresponding octamides, decamides, dodecamides, and tetradecamides. The protecting groups were then removed and the oligoamines were isolated as hydrochlorides. In a typical reaction, a tetramide (3 mmol) and the butenesulfonate (1.5 mmol) were mixed in 20 ml of dimethylformamide kept at 5°C, sodium hydride (3.6 mmol) was added, and the mixture was kept at 20°C for 18 h. The solvent was evaporated; the residue was partitioned between chloroform and a concentrated ammonium chloride solution; and the product, extracted into the organic layer, was purified by flash chromatography on silica gel (hexane-ethyl acetate [6:4]). The octamide thus obtained (83% yield) was deprotected by dissolution (0.50 mmol) in methylene chloride (20 ml), followed by addition of phenol (37 mmol) and 33% hydrogen bromide in glacial acetic acid (20 ml). The mixture was kept at 20°C for 18 h; further workup was by previously published procedures (32). Octamines SL-11157 and SL-11158 were thus obtained (as their hydrochlorides) in 89% yields. Reduction of the double bond was achieved by hydrogenation of an aqueous solution of either SL-11158 or SL-11157 over platinum oxide at 50 lb/in2 for 2 h; SL-11160 was thus obtained in an 85% yield. Analytical data supported the assigned structures.
FIG. 2.
Structures of polyamine analogues (tetramines and pentamines) with restricted conformations.
FIG. 3.
Structures of oligoamines and pentamidine analogues.
The three pentamidine analogues (SL-11134, SL-11136, and SL-11137) were obtained by modifying known procedures. Thus, 1,5-bis(p-cyanobenzyl)-diaminobutane, 1,6-bis(p-cyanobenzyl)-diaminopentane, and 1,7-bis(p-cyanobenzyl)-diaminohexane were obtained by previously described procedures (33). The corresponding amidines were prepared by the following procedure. 1,6-Bis(p-cyanobenzyl)-diaminopentane (1 mmol) was dissolved in 6 ml of anhydrous tetrahydrofuran under argon, lithium hexamethyl disilazide (4 mmol) dissolved in tetrahydrofuran was added, and the mixture was stirred at 20°C for 2 h. It was then cooled at 5°C; 10 ml of 1 M hydrogen chloride in ether was added; and the white precipitate was filtered, washed with ether, and crystallized from ethanol-ether; 0.5 g (94% yield) of SL-11134 tetrahydrchloride was obtained. Analytical data supported the assigned structures.
E. cuniculi culture and drug assay.
E. cuniculi culture and the drug assay were performed as described previously (1). RK-13 cells (5 × 104 cells/well) were plated into each well of a Falcon multiwell, 24-well tissue culture plate and were allowed to incubate for 3 days until they were confluent (8). These cells were then infected with spores of E. cuniculi at a multiplicity of infection of 4:1 (106 organisms per well). This protocol resulted in infection of 50 to 80% of cells in the absence of drug treatment. Drugs were added to duplicate wells and the plates were incubated for 7 days, with the drug-containing medium changed at 3 and 6 days. On day 8, the wells were fixed overnight and stained with Giemsa, and the cells were counted with an inverted microscope at ×400 magnification. Five confluent fields (240 cells/field) were counted for control as well as for drug-treated wells. The percentage of infected cells in the presence of the compound was compared to the percentage of infected control cells. Fifty percent inhibitory concentrations (IC50s) were expressed as micromolar of drug (8). Toxicity to the host cell monolayer was determined by examination of Giemsa-stained, uninfected, drug-treated monolayers for abnormal morphology, such as deformed fibroblasts, ragged holes in the monolayer, detachment of the monolayer, and evidence of lysis.
Mouse model of microsporidiosis.
Two well-validated models of microsporidiosis were used, one with nude (nu/nu) BALB/c mice (10) which are completely immunosuppressed and the other with C57BL/6J CD8-knockout (ΔCD8) mice (19). The latter immune defect is specific, allowing growth of microsporidia. ΔCD8 mice (19) were infected with 3 × 107 spores (intraperitoneally [i.p.]) 24 h before treatment, and nude mice were infected with 106 spores (i.p.) 24 h before treatment (10). Animals were treated i.p. with 1 to 10 mg of drug per kg of body weight for 5 days, followed by 2 days without drug and then 5 more days of drug treatment. This protocol has been used for the administration of these compounds in animal tumor models and for initial toxicity testing. Animals were considered cured of infection if they survived more than 28 days postinfection with no evidence of the presence of microsporidia. Tissues were obtained from mice at the ends of the observation periods and were then fixed and embedded by standard protocols. Tissue sections were stained for microsporidia with hematoxylin-eosin and tissue chromotrope stains. These sections were examined in a blinded fashion for pathology and the presence of organisms.
PCR assay for microsporidia.
As published previously (27), the parasite loads in the tissues were estimated by using a semiquantitative PCR with DNA extracted from tissues (liver and kidney) at the termination of each experiment. DNA was extracted by use of the Qiamp tissue kit (Qiagen, Chatsworth, Calif.), and 3 μg of each sample was analyzed. The PCR was performed with a pair of primers, 5′-ATGAGAAGTGATGTGTGCG-3′ and 5′-TGCCATGCACTCACAGGCATC-3′, that amplify a 549-bp fragment of the small-subunit rRNA gene of E. cuniculi (GenBank accession no. L17072). A 510-bp competitive internal standard was generated by the method of Kirisits et al. (21), as reported previously (27). PCR was performed using the following conditions: 35 cycles of denaturation at 94°C for 45 s, annealing at 53°C for 1 min, and elongation at 72°C for 30 s. Amplification was performed with the Eppendorf Scientific Inc., Westbury, N.Y.) master kit and with dGTP, dATP, dTTP, and dCTP each at a concentration of 0.2 mM and each E. cuniculi-specific primer at a concentration of 0.4 μM. Various amounts of the internal standard were added to each reaction mixture to determine the relative amounts of parasite rRNA in each sample. Amplicons were analyzed by electrophoresis on a 1.5% agarose gel and were visualized with ethidium bromide. The number of parasites was determined by amplification of a known amount of parasite with a dilution of internal standard by using the same PCR conditions described above (27). The internal standard was used as a quality control to determine the ratios of the amount of competitive template-directed product to the amount of microsporidian template-directed product.
Toxicity studies of polyamine analogues in mice.
All agents were administered i.p. for 5 days, followed by 2 days without drug and then another 5 days of treatment. The mice (groups of three mice each) weighed 23 to 33 g and were matched as to approximate weight. The weight loss 1 day after the end of treatment is expressed in comparison to the weight at the start of treatment. Control (untreated) animals gained 1 to 3 g in 14 days, depending on their starting weight. Daily observations on clinical toxicity (e.g., decreased movement, ruffled fur, and decreased grooming) were obtained for all animals. The general dose range used was 1 to 150 mg/kg/day, depending on the agent.
RESULTS
Polyamine analogs.
Four classes of polyamine analogues were evaluated for activity against E. cuniculi, the species used in two murine models of microsporidiosis. The first class comprises the tetramines (Fig. 2), homologues of spermine in which the external aminopropyl residues present in spermine (Fig. 1) were replaced by aminobutyl residues, making a homospermine backbone. The terminal primary amino groups were N-ethylated to prevent oxidation by serum aminooxidases (6). Spermine and homospermine are freely rotating molecules that can assume myriad conformations. The introduction of alicyclic residues in the homospermine backbone, as in SL-11093, SL-11098, SL-11099, and SL-11100 (Fig. 2), restricts free rotation at the central segment of the molecule. This is also the case when one or two cis double bonds are introduced, as in SL-11102, SL-11114, SL-11118, and SL-11119. The introduction of a triple bond or a 1,2-dimethylbenzene residue in the central segment, as in SL-11103 and SL-11104, respectively, confers rigidity to that part of the molecule. Spermine and its homologues are regioselective binders (i.e., they bind selectively to a domain or region) of DNA (12), tRNA (15), chromatin (4), and rRNA (7). Spermine analogues that are conformationally restricted inhibit tumor cell proliferation (32), very likely by bending and kinking the conformations of the nucleic acids to which they bind. The potential utility of conformationally restricted homospermine analogues as inhibitors of replication of microsporidia was therefore explored.
The pentamines (Fig. 2) were the second class of polyamine analogs investigated. Pentamine SL-11061 (also called BE-4-4-4-4), designed in our laboratories several years ago, is a powerful inhibitor of tumor cell replication (3). Its free rotating conformation was also restricted by the introduction of cis double bonds into its hydrocarbon skeleton, as in SL-11121 to SL-11123 and SL-11126 to SL-11130.
Hydrophilic groups were also introduced into the pentamine structure: an alcohol residue in SL-11141 and a nonchiral amino acid in SL-11143.
The third class of polyamine analogues used in the present study consisted of the oligoamines, SL-11144, SL-11157, SL-11158, SL-11159, SL-11160, SL-11172, SL-11175, and SL-11207 (Fig. 3). We coined the name oligoamines for this new class of synthetic octa-, deca-, dodeca-, and tetradecamines since the chemically more correct name of “polyamines” had historically been applied to the natural tetra-, tri-, and diamines (Fig. 1). The rationale behind the synthesis of oligoamines is the well-known fact that spermine (a tetramine), at a concentration range of 0.05 to 0.1 mM and at near physiological ionic strength, leads to the collapse of DNA (29). Oligoamines were found to condense DNA at much lower concentrations (2 to 4 μM) and are indeed powerful inhibitors of human tumor cell proliferation.
The fourth class of polyamine derivatives tested consisted of three diamino analogs of pentamidine (SL-11134, SL-11136, and SL-11137; Fig. 3). Pentamidine is a well-known antiprotozoal drug (7).
In vitro susceptibilities to polyamine analogs.
The results of the in vitro screening are summarized in Table 1. E. cuniculi was used as a model organism for determination of the antimicrosporidial activities of the polyamine analogs. It is not feasible to screen agents directly for activity against E. bieneusi due to the absence of in vitro cultivation systems or small animal models. E. cuniculi has been reported in cases of encephalitis, disseminated infection, and hepatitis in humans (44). In previous work (8, 44), other compounds screened have demonstrated similar inhibition profiles against all of the Encephalitozoonidae. In vitro activity was assayed as described previously (8). Polyamine analogs were added to E. cuniculi-infected RK-13 cells, and the cultures were incubated for 7 days, with the drug-containing media changed on days 3 and 6. The percentages of infected cells containing clusters of parasites in treated and untreated cultures were compared.
TABLE 1.
Activities of polyamine analogs against E. cuniculi in vitro
| Compound | IC50 (μM) | IC50 range (μM) | No. of trials |
|---|---|---|---|
| SL-11093 | 270 | 220–340 | 2 |
| SL-11094 | 95 | 88–102 | 3 |
| SL-11098 | 166.5 | 103–230 | 2 |
| SL-11099 | 325 | 320–330 | 2 |
| SL-11100 | 3,800 | 1 | |
| SL-11102 | 118 | 1 | |
| SL-11103 | 9,000 | 1 | |
| SL-11104 | 3,700 | 1 | |
| SL-11114 | 600 | 1 | |
| SL-11118 | 745 | 490–1000 | 2 |
| SL-11061 | 42.5 | 42–43 | 2 |
| SL-11119 | 410 | 330–490 | 2 |
| SL-11121 | 137.5 | 95–180 | 2 |
| SL-11122 | 600 | 1 | |
| SL-11123 | 500 | 1 | |
| SL-11124 | 265 | 250–280 | 2 |
| SL-11126 | >1,000 | 2 | |
| SL-11127 | >1,000 | 2 | |
| SL-11128 | >1,000 | 2 | |
| SL-11129 | 500 | 1 | |
| SL-11130 | 385 | 330–440 | 2 |
| SL-11132 | 1,400 | 1 | |
| SL-11141 | 62.5 | 24–91 | 6 |
| SL-11143 | 58.5 | 50–75 | 4 |
| SL-11134 | 148 | 50–330 | 3 |
| SL-11136 | 95 | 50–160 | 3 |
| SL-11137 | 317 | 100–580 | 4 |
| SL-11157 | 1.9 | 1.2–2.7 | 4 |
| SL-11158 | 8.2 | 1.1–22 | 3 |
| SL-11144 | 0.62 | 0.34–0.76 | 4 |
| SL-11159 | 0.63 | 0.56–0.68 | 2 |
| SL-11160 | 1.67 | 1.35–2.0 | 2 |
| SL-11172 | 0.40 | 0.19–0.60 | 2 |
| SL-11175 | 0.54 | 0.50–0.58 | 2 |
| SL-11207 | 30.3 | 20.2–40.8 | 2 |
The analogues in the first class, namely, the conformationally restricted tetramines SL-11093, SL-11098 to SL-11100, SL-11102 to SL-11104, SL-11114, SL-11118, and SL-11119, were minimally active. With the exception of the fully saturated derivative SL-11061, the analogues in the second class, the pentamines SL-11061, SL-11121 to SL-11123, SL-11126 to SL-11130, SL-11141, and SL-11143, were not very active either; SL-11061, however, inhibited parasite replication at relatively low concentrations (IC50, 42 to 43 μM). The pentamidine analogues (SL-11134, SL-11136, and SL-11137) were also inactive. In contrast, the oligoamines seemed to hold promise as antimicrosporidial agents. The octamines (SL-11157, SL-11158, and SL-11160) displayed low IC50s, the decamines (SL-11144 and SL-11159) were even more active, while the dodecamine (SL-11172) and the tetradecamine (SL-11175) were also very active in the inhibition of parasite growth.
Interestingly, SL-11207, although a nonamine, was effective only at a somewhat higher range of IC50s, similar to those of SL-11061. This could be because the more active oligoamines are a continuous catenation of NH2+ groups (polyamines are protonated at neutral pH) that can wrap and bind (either by charge or by hydrogen bonds [17]) to DNA and cause it to collapse. In SL-11207, the polycationic chain is interrupted by a nonprotonated tertiary amine residue and therefore might not interact with the phosphate groups of DNA in the correct manner. The protons of two NH2+ residues separated by a zig-zag chain of four methylene groups are at a distance of 7.33 Å from each other, a value that fits the distance between successive phosphate anions in the DNA helix (7.3 Å), as well as the distance between phosphate groups (7.96 Å) found in spermine phosphate crystals (36).
Toxicity studies.
In tandem with the in vitro studies, we determined the tolerance of select polyamine analogues by mice as a prelude to in vivo studies. The data are shown in Table 2. SL-11061 was tolerated up to at least 10 mg/kg when it was given i.p. daily for 5 days, followed by 2 days with no treatment and then a second 5-day course of therapy. Animals were observed for weight loss and lethality for at least 20 days following drug injection. Host tolerances of the oligoamines SL-11158 and SL-11144 were similar (Table 2). Polyamines with alicyclic rings, e.g., SL-11093 and SL-11099, were tolerated up to 50 mg/kg when the schedule described above was used. The weight loss in animals treated with SL-11093 was negligible.
TABLE 2.
Relative host tolerance of polyamine analogs
| Dose (mg/kg) | No. of deaths/group | Day of death | Avg weight loss or gain (g) 1 day posttreatment |
|---|---|---|---|
| SL-11061 | |||
| 0 | 0 | +1.7 | |
| 1 | 0 | +0.8 | |
| 2.5 | 0 | 0 | |
| 5 | 0 | −0.1 | |
| 10 | 0 | +0.7 | |
| SL-11091 | |||
| 0 | 0 | +1.4 | |
| 5 | 0 | +2.6 | |
| 10 | 0 | +2.4 | |
| 15 | 0 | +1.0 | |
| 20 | 0 | +1.9 | |
| 25 | 0 | +1.1 | |
| SL-11093 | |||
| 0 | 0 | +4.1 | |
| 5 | 0 | +2.2 | |
| 10 | 0 | +2.4 | |
| 15 | 0 | +2.8 | |
| 20 | 0 | +1.6 | |
| 25 | 0 | +2.2 | |
| 50 | 0 | −0.1 | |
| 100 | 2 | 5, 8 | −6.5 |
| 150 | 3 | 4, 8, 8 | |
| SL-11099 | |||
| 0 | 0 | +0.45 | |
| 1 | 0 | −1.17 | |
| 2.5 | 0 | +1.16 | |
| 5 | 0 | 0 | |
| 10 | 0 | −0.58 | |
| 15 | 0 | +0.16 | |
| 25 | 0 | −1.34 | |
| 50 | 0 | −2.34 | |
| SL-11144 | |||
| 0 | 0 | +1.7 | |
| 0 | 0 | +4.1 | |
| 1 | 0 | +1.5 | |
| 2.5 | 0 | −0.9 | |
| 5 | 0 | +0.2 | |
| 10 | 0 | +0.3 | |
| 15 | 3 | 9, 9, 15 | |
| 25 | 3 | 3, 3, 3 | |
| SL-11158 | |||
| 0 | 0 | +1.7 | |
| 1 | 0 | +1.5 | |
| 2.5 | 0 | +1.0 | |
| 5 | 0 | +0.7 | |
| 10 | 0 | +0.1 | |
| 15 | 1 | 8 | −1.5 |
| 25 | 3 | 3, 3, 3 |
Efficacies of polyamine analogs in experimental infections.
In vivo therapeutic studies were then undertaken with the same strain of E. cuniculi that was used in vitro. The studies were carried out in separate laboratories with C57BL/6J ΔCD8 mice in one laboratory and BALB/c nude (nu/nu) mice in the other (Table 3) (10, 19). In these studies, immunodeficient mice were inoculated (i.p.) with 1 × 106 to 3 × 107 spores and treated (i.p.) with the treatment schedule described above, beginning 24 h after infection. SL-11061 at 1.25 mg/kg or at 10 mg/kg/day cured 10 of 11 BALB/c nu/nu animals, with 1 animal dying of infection on day 20 (Table 3, experiments 1 and 2), and SL-11061 at 1.25 mg/kg cured 2 of 2 C57BL/6J ΔCD8 animals (Table 3, experiment 4). The oligoamines were even more effective. SL-11144 was curative at 1.25 mg/kg (two of two animals) or at 2.5 mg/kg/day (three of three animals), while SL-11158 at 5 mg/kg/day cured four of four animals (Table 3, experiments 3, 4, and 5). In view of the very low toxicity of SL-11093, it was also assayed in vivo. Even though it had only moderate activity in vitro (Table 1), it was curative in vivo when it was given at 5 mg/kg/day by the same treatment schedule mentioned above; it cured five of five animals. Animals considered cured in these studies were observed for evidence of parasitemia for 30 days after untreated control animals died and were also examined for evidence of parasites in liver by histologic staining as well as by PCR (by a semiquantitative assay for E. cuniculi [27]), with no parasites being observed by either technique.
TABLE 3.
Effects of polyamine analogs on mice infected with E. cuniculi
| Expt no. and mouse strain | Treatment (dose [mg/kg]) | No. of mice with ascites/no. of mice tested | No. of deaths/no. of mice tested | Cure rate (%) |
|---|---|---|---|---|
| Expt 1, BALB/c nu/nu | Control | 6/6 | 6/6a | |
| SL-11061 (BE-4-4-4-4) (10) | 1/6 | 0/6b | 100 | |
| Expt 2, BALB/c nu/nu | Control | 6/6 | 6/6c | |
| SL-11061 (10) | 5/5 | 1/5d | 80 | |
| Expt 3, C57BL/6J ΔCD8 | Control | 3/3 | 3/3e | |
| SL-11144 (2.5) | 0/3 | 0/3 | 100 | |
| Expt 4, C57BL/6J ΔCD8 | Control | 2/2 | 2/2e | |
| SL-11144 (1.25) | 0/2 | 0/2 | 100 | |
| SL-11061 (1.25) | 0/2 | 0/2 | 100 | |
| Expt 5, C57BL/6J ΔCD8 | Control | 2/2 | 2/2e | |
| SL-11158 (5) | 0/4 | 0/4 | 100 | |
| Expt 6, C57BL/6J ΔCD8 | Control | 5/5 | 5/5f | |
| SL-11093 (1.0) | 5/5 | 1/5d | 80 | |
| SL-11093 (5.0) | 0/5 | 0/5 | 100 |
All mice were dead by day 27.
No deaths during a 50-day observation period.
All mice were dead by day 49; cured animals were observed for 20 days after deaths of control mice.
All mice were dead by day 20.
All mice were dead by day 14.
All mice were dead by day 22. None of the treated mice had ascites, and there was no evidence of the organism by PCR analysis (27) or histologic staining tissues in cured mice.
DISCUSSION
Binding to nucleic acids is very likely the most important biological function of polyamines. Binding of these compounds to DNA and chromatin (4, 12), tRNA (15–17), and RNA (5) results from electrostatic forces as well as from hydrogen-bonding interactions that arise from the protonated secondary nitrogens on the polyamines and acceptor residues on the nucleic acids. The binding of synthetic polyamine analogues to nucleic acids provides the most likely explanation for their strong antiproliferative effects (14, 24) since the structural distortions that they introduce in nucleic acids impair the latter’s biological functions (12). In this report we have shown that several synthetic polyamine analogues are efficient antimicrosporidial agents with good host tolerance and with low toxicity at effective doses. The efficacies of the analogues in controlling microsporidosis can be attributed to the combination of the unique life cycle pattern of the microsporidia and the affinities of polyamines for binding to nucleic acids.
In the infective phase, the spores of the microsporidia pierce the host cell plasmalemma by extruding into the host cell the polar tubule through which the microsporidian sporoplasm is injected into the host cell. Massive arrays of ribosomes surround the developing polar filament coils in immature spores (1). Ribosomes are also present in the sporoplasm that mature spores inject into the host cell, and they are a predominant component of the microsporidian cytoplasm. These abundant ribosomes are not of the typical eukaryotic type but resemble the ribosomes of prokaryotic organisms and have a very high rate of protein synthesis during the initial infective cycle. Within the first 48 h after the sporoplasm has reached the host cell, several rounds of division and even spore formation may occur (40).
Prokaryotic ribosomes contain up to 15% polyamines (mainly spermine and spermidine), which help maintain the relatively compact structure of the ribosome (7). They are bound to the rRNA, which makes up 70% of the ribosomal mass. Interestingly, despite their binding to RNA, the exchange of ribosomal polyamines with the media is a function of the polyamines present in the media (35). The importance of polyamines for ribosomal function can be inferred from the emerging evidence that shows that most of the activities of the ribosome, i.e., catalysis, peptide bond formation, decoding, and ribosomal translocation, are performed by RNA itself (2, 28). It is therefore highly likely that in ribosome-rich parasites such as microsporidia, which inject highly active parasite ribosomal clusters into the host cell, external polyamine analogues exchange with the natural ribosomal polyamines and bind to the RNA chains, thus impairing their catalytic activities. Because of the rather unexpected finding that RNA domains are the catalytic centers of the ribosomes, the use of polyamine analogues to target rRNA has become a rationale approach for future drug design.
While the oligoamines SL-11158 and SL-11144 are active against E. cuniculi both in vitro and in vivo (Tables 1 and 3), the tetramine SL-11093 and the pentamine SL-11061 are weak or poor inhibitors of parasite growth in vitro (Table 1). Both of these compounds were, however, found to be active inhibitors in vivo (Table 3). The latter results illustrate one of the hurdles of medicinal chemistry, i.e., that in successful drug design absorption and pharmacokinetics are as important as target binding (39). SL-11144, SL-11158, SL-11093, and SL-11061 very likely have the correct biopharmaceutical properties that make them eligible as promising antimicrosporidial drugs. Recent work has shown that the large oligoamines (SL-11158 and SL-11144) are avidly taken up by cancer cells, while the shorter molecules (SL-11061 and SL-11093) have a short plasma half-life (B. Frydman, unpublished data).
Inasmuch as microsporidial infections are emerging infectious diseases and are still an important problem in patients with AIDS, as well as in patients with other immunocompromised states, continued study of select polyamine analogs with a focus on the initiation of human clinical trials appears to be justified.
Acknowledgments
This work was supported in part by the National Institutes of Health through grants AI 41398 (to M.W.), AI43693 (to A.I.K.), and AI 43094 (to B.F.), as well as a Pace University Scholarly Research Award (to C.J.B.).
We thank Jenny Gallardo, Lakshman Mazumder, and Elvis Rosero for technical help.
REFERENCES
- 1.Bacchi, C. J., S. Lane, L. M. Weiss, N. Yarlett, P. Takvorian, and M. Wittner. 2001. Polyamine synthesis and interconversion by the microsporidian Encephalitozoon cuniculi. J. Eukaryot. Microbiol. 48:374–381. [DOI] [PubMed] [Google Scholar]
- 2.Ban, N., P. Nissen, J. Hansen, P. B. Moore, and T. A. Steitz. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289:905–920. [DOI] [PubMed] [Google Scholar]
- 3.Basu, H. S., M. Pellarin, B. G. Feuerstein, A. Shirahata, K. Samejima, D. F. Deen, and L. J. Marton. 1993. Interaction of a polyamine analogue, 1,19-bis-(ethylamino)-5,10-15-triazanonadecane (BE-4-4-4-4), with DNA and effect on growth, survival, and polyamine levels in seven human brain tumor cell lines. Cancer Res. 53:3948–3955. [PubMed] [Google Scholar]
- 4.Basu, H. S., I. V. Smirnov, H. F. Peng, K. Tiffany, and V. Jackson. 1997. Effects of spermine and its cytotoxic analogues on nucleosome formation in topologically stressed DNA in vitro. Eur. J. Biochem. 243:247–258. [DOI] [PubMed] [Google Scholar]
- 5.Bolton, P. H., and D. R. Kearns. 1978. Hydrogen bonding interactions of polyamines with the 2′OH of RNA. Nucleic Acids Res. 5:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, S. S. 1998. A guide to the polyamines, p.69–93. Oxford University Press, New York, N.Y.
- 7.Cohen, S. S. 1998. A guide to the polyamines, p.105–106 and 271–272. Oxford University Press, New York, N.Y.
- 8.Coyle, C., M. Dent, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1998. TNP-470 is an effective antimicrosporidial agent. J. Infect. Dis. 177:515–518 [DOI] [PubMed] [Google Scholar]
- 9.Deplazes, P., A. Mathis, and R. Weber. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 6:236–260. [DOI] [PubMed] [Google Scholar]
- 10.Didier, E. S., P. W. Varner, P. J. Didier, A. M. Aldras, N. J. Millichamp, and M. Murphey-Corb. 1994. Experimental microsporidiosis in immunocompetent and immunodeficient mice and monkeys. Folia Parasitol. 41:1–11. [PubMed] [Google Scholar]
- 11.Dieterich, D. T., E. A. Lew, K. P. Kotler, M. A. Poles, and J. M. Orenstein. 1994. Treatment with albendazole for intestinal disease due to Enterocytozoon bieneusi in patients with AIDS. J. Infect. Dis. 169:178–183. [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein, B. G., L. D. Williams, H. S. Basu, and L. J. Marton. 1991. Implications and concepts of polyamine-nucleic acid interactions. J. Cell. Biochem. 6:37–47. [DOI] [PubMed] [Google Scholar]
- 13.Franssen, F. F., J. T. Lumeij, and F. van Knapen. 1995. Susceptibility of Encephalitozoon cuniculi to several drugs in vitro. Antimicrob. Agents Chemother. 39:1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frydman, B., and A. Valasinas. 1999. Polyamine-based chemotherapy of cancer. Exp. Opin. Ther. Patents 9:1055–1068. [Google Scholar]
- 15.Frydman, B., W. M. Westler, and K. Samejima. 1996. Spermine binds in solution to the TΨC loop of t-RNAPhe: evidence from a 750 MHz 1H-NMR analysis. J. Org. Chem. 61:2588–2589. [DOI] [PubMed] [Google Scholar]
- 16.Frydman, B., W. M. Westler, A. Valasinas, D. L. Kramer, and C. W. Porter. 1999. Regioselective binding of spermine, 1N,12N-bismethylspermine, and 1N,12N-bisethylspermine to t-RNAPhe as revealed by 750 MHz 1H-NMR and its possible correlation with cell cycling and cytotoxicity. J. Braz. Chem. Soc. 10:241. [Google Scholar]
- 17.Frydman, L., P. C. Rossomando, V. Frydman, C. O. Fernandez, B. Frydman, and K. Samejima. 1992. Interaction of natural polyamines with t-RNA: a 15N-NMR study. Proc. Natl. Acad. Sci. USA 89:9186–9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbo, T., S. Sarbah, I. T. Gangaidzo, Y. Ortega, C. R. Sterling, A. Carville, S. Tzipori, and P. M. Wiest. 1999. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS 13:819–821. [DOI] [PubMed] [Google Scholar]
- 19.Kahn, I. A., J. D. Schwartzman, L. H. Kasper, and M. Moretto. 1999. CD8+ CTLs are essential for protective immunity against Encephalitozoon infection. J. Immunol. 162:6086–6091. [PubMed] [Google Scholar]
- 20.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with β-tubulin sequence. Antimicrob. Agents Chemother. 38:2086–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirisits, M., E. Mui, and R. McLeod. 2000. Measurement of the efficacy of vaccines and antimicrobial therapy against infection with Toxoplasma gondii. Int. J. Parastiol. 30:149–155. [DOI] [PubMed] [Google Scholar]
- 22.Kotler, D. P., and J. M. Orenstein. 1999. Clinical syndromes associated with microsporidosis, p.258–292. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidosis. American Society for Microbiology, Washington, D.C.
- 23.Li, Y., J. L. Eiseman, D. L. Sentz, F. A. Rogers, S. S. Pan, L.-T. Hu, M. J. Egorin, and P. S. Callery. 1996. Synthesis and antitumor evaluation of a highly potent cytotoxic DNA cross-linking polyamine analogue, 1,12-diaziridinyl-4,9-diazadodecane. J. Med. Chem. 39:339–341. [DOI] [PubMed] [Google Scholar]
- 24.Marton, L. J., and A. E. Pegg. 1995. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 35:55–91. [DOI] [PubMed] [Google Scholar]
- 25.Matsubayashi, H., T. Koike, L. Mikata, H. Takei, and, S. A. 1959. A case of an encephalitozoon-like body in man. Arch. Pathol. 67:181–187. [PubMed] [Google Scholar]
- 26.Molina, J. M., J. Goguel, C. Sarfati, C. I. Chastang, I. Desporte-Livage, J. F. Michiels, C. Maslo, C. Katlama, L. Cotte, C. Leport, F. Raffi, F. Deroain, and J. Modai. 1997. Potential efficacy of fumagillin in intestinal microsporidiosis due to Enterocytozoon bieneusi in patients with HIV infection: results of a drug screening study. AIDS 11:1603–1610. [DOI] [PubMed] [Google Scholar]
- 27.Moretto, M., L. Casciotti, B. Durell, and I. A. Khan. 2000. Lack of CD4+ T cells does not affect induction of CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect. Immun. 68:6223–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nissen, P., J. Hansen, N. Ban, P. B. Moore, and T. A. Steitz. 2000. The structural basis of ribosome activity in peptide bond synthesis. Science 289:920–930. [DOI] [PubMed] [Google Scholar]
- 29.Osland, A., and K. Kleppe. 1977. Polyamine induced aggregation of DNA. Nucleic Acids Res. 4:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastrelli, P. D., E. S. Didier, and R. W. Yee. 1994. Microsporidial keratitis. Ophthalmol. Clin. N. Am. 7:617–633. [Google Scholar]
- 31.Reddy, V. K., A. Sarkar, A. Valasinas, L. J. Marton, H. S. Basu, and B. Frydman. 2001. cis-Unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cell lines. J. Med. Chem. 44:404–417. [DOI] [PubMed] [Google Scholar]
- 32.Reddy, V. K., A. Valasinas, A. Sarkar, H. S. Basu, L. J. Marton, and B. Frydman. 1998. Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cells. J. Med. Chem. 41:4723–4732. [DOI] [PubMed] [Google Scholar]
- 33.Samejima, K., N. Matsushima, M. Miitsu, and B. Frydman. 1995. Synthesis of [5,8-13C2]- and [1,12-13C2]-spermines using potassium [13C]-cyanide as [13C] source. Chem. Pharm. Bull. 43:2001. [Google Scholar]
- 34.Sprague, V., and J. Vavra. 1977. Systematics of microsporidia, p.2. In L. A. Bulla and T. C. Cheng (ed.), Comparative pathobiology. Plenum Press, New York, N.Y.
- 35.Tabor, C. W., and P. D. Kellogg. 1967. The effect of isolation conditions on the polyamine content of Escherichia coli ribosomes. J. Biol. Chem. 24:1044–1052. [PubMed] [Google Scholar]
- 36.Tsuboi, S. 1964. On the melting temperature of nucleic acid in solution. Bull. Chem. Soc. Jpn. 37:1514. [Google Scholar]
- 37.Valasinas, A., A. Sarkar, V. K. Reddy, L. J. Marton, H. S. Basu, and B. Frydman. 2001. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 44:390–403. [DOI] [PubMed] [Google Scholar]
- 38.Valperga, S. M., S. A. de Jogna Prat, G. J. de Valperga, S. G. Lazarte, A. V. de Trejo, N. Diaz, and H. M. Huttman. 1999. Microsporidian spores in the stool specimens of toddlers with or without diarrhea from Tucuman, Argentina. Rev. Argent. Microbiol. 31:157–164. [PubMed] [Google Scholar]
- 39.van de Waterbeemd, H., D. A. Smith, K. Beaumont, and D. K. Walker. 2001. Property-based design: optimization of drug absorption and pharmacokinetics. J. Med. Chem. 4:1313–1333. [DOI] [PubMed] [Google Scholar]
- 40.Vavra, J., and J. I. R. Larsson. 1999. Structure of the microsporidia, p.7–84. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidosis. American Society for Microbiology, Washington, D.C.
- 41.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss, L. M., E. Michalakakis, C. M. Coyle, H. B. Tanowitz, and M. Wittner. 1994. The activity of albendazole against Encephalitozoon cuniculi. J. Eukaryot. Microbiol. 41:65S. [PubMed] [Google Scholar]
- 43.Wittner, M., H. B. Tanowitz, and L. M. Weiss. 1983. Parasitic infections in AIDS patients: cryptosporidiosis, isopsoriasis, microsporidosis, cyclosporiasis. Infect. Dis. Clin. N. Am. 7:569–586 [PubMed] [Google Scholar]
- 44.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. American Society for Microbiology, Washington, D.C.