Abstract
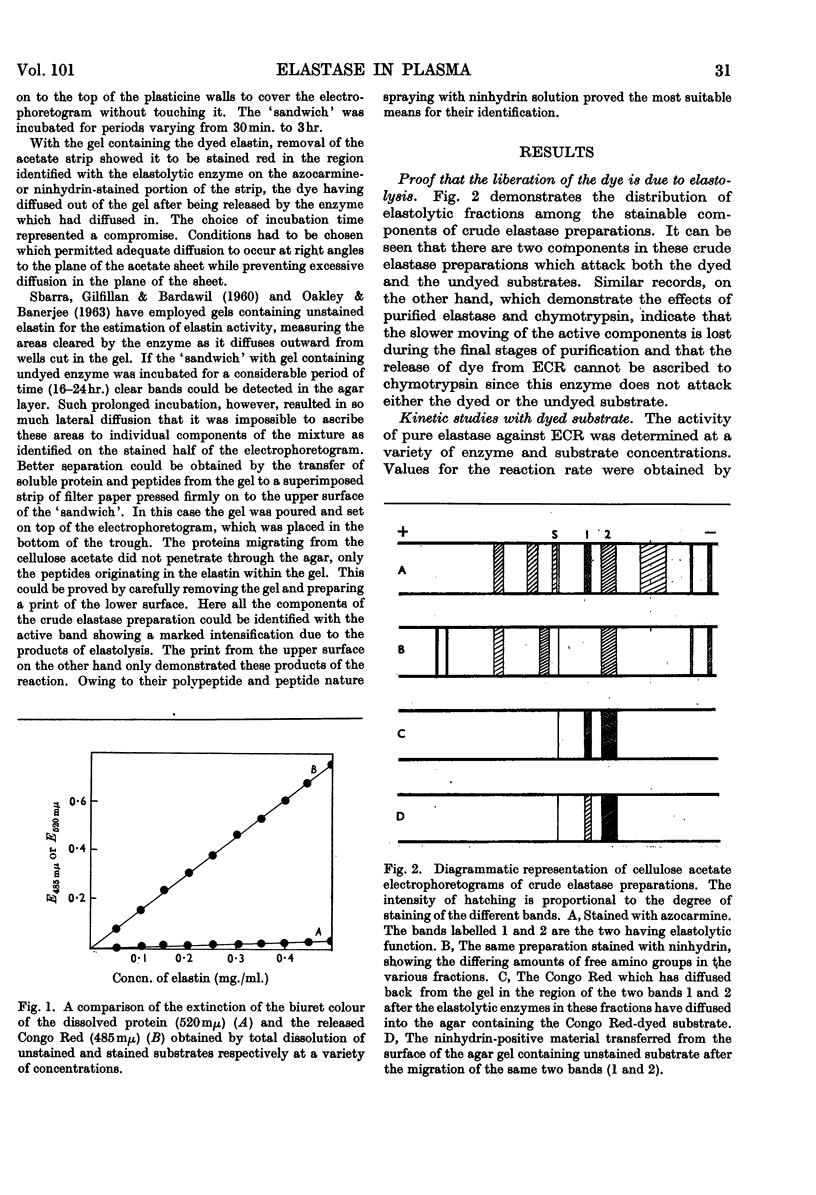
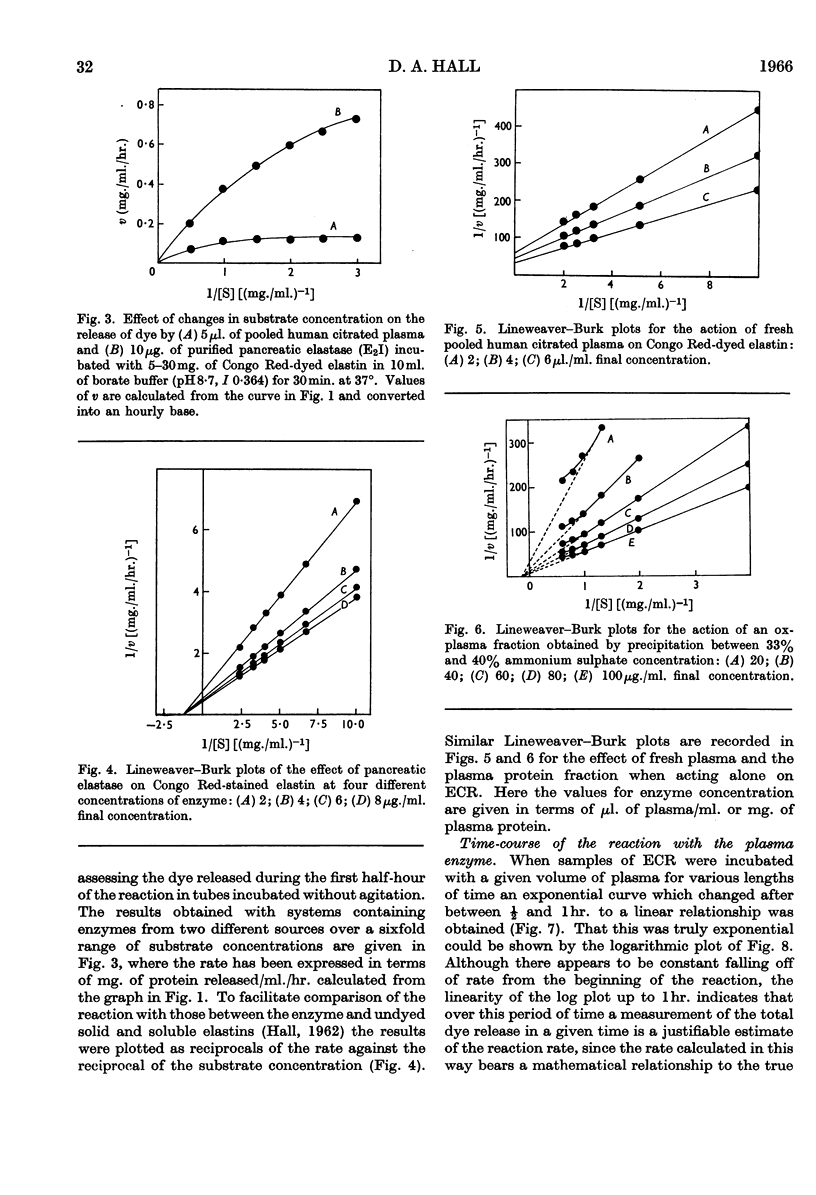
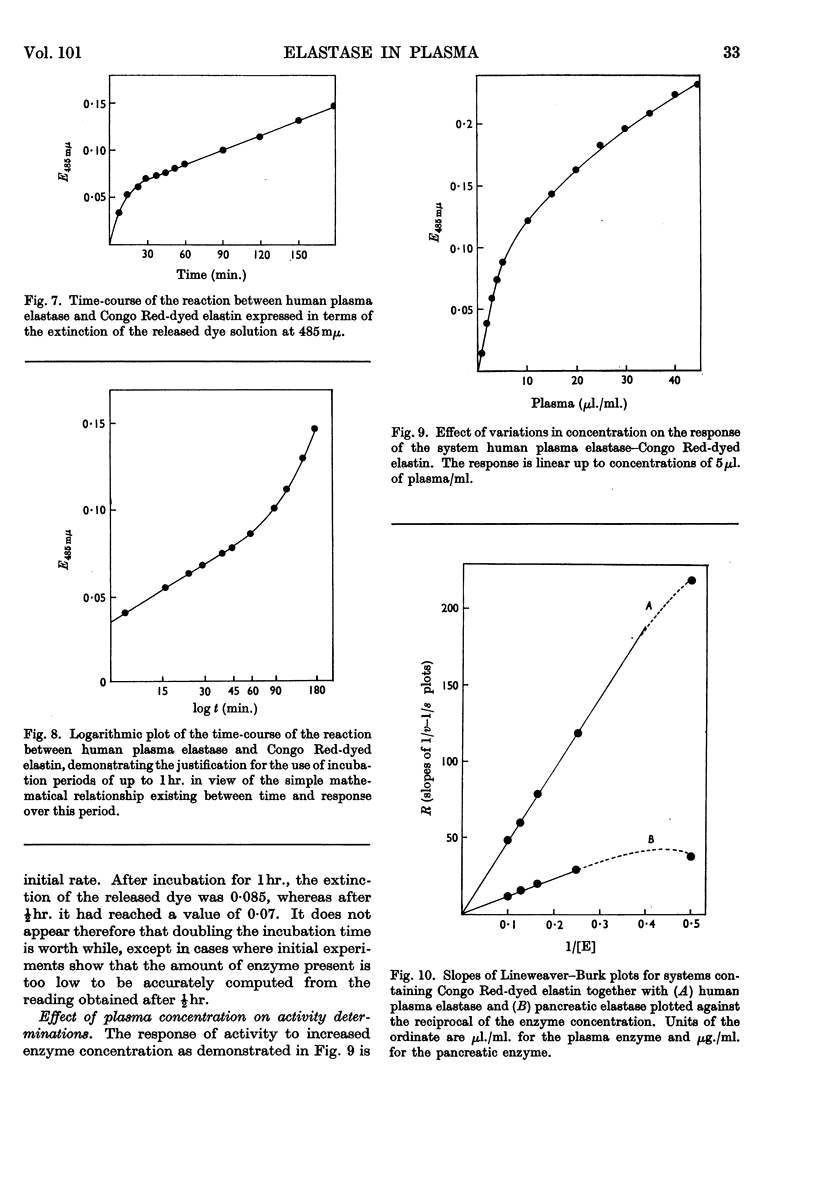
1. Electrophoretic separation of partially purified elastase preparations from pancreas followed by incubation of the electrophoretogram in contact with an agar gel containing 4% of either Congo Red-stained or unstained elastin demonstrated that the enzyme which dissolves elastin can be identified with that which releases dye from the stained preparation. 2. A method for the estimation of elastase based on the release of dye from Congo Red-stained elastin is described. It is 23 times as sensitive as methods employing protein determination. 3. With the method, elastase activity can be identified in plasma and in a partially fractionated plasma protein preparation. 4. Lineweaver–Burk plots of these estimates of activity at a variety of substrate concentrations indicate that the reaction between elastase and the dyed elastin is more closely similar to that between elastase and the soluble substrate elastin rather than to that between elastase and the solid substrate. 5. Values for Km and Vmax. calculated for the enzyme present in plasma present further evidence for its identity with the pancreatic enzyme. 6. By calculation of the slopes of the Lineweaver–Burk plots for various enzyme concentrations it has proved possible to demonstrate that the inhibitor which is also present in the plasma is without effect on the enzyme when it acts on the dyed substrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALO J., BANGA I. The elastolytic activity of pancreatic extracts. Biochem J. 1950 Apr;46(4):384–387. doi: 10.1042/bj0460384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALO J. CONNECTIVE TISSUE CHANGES IN ATHEROSCLEROSIS. Int Rev Connect Tissue Res. 1963;1:241–306. [PubMed] [Google Scholar]
- BANGA I., BALO J., HORVATH M. Nephelometric determination of elastase activity and method for elastoproteolytic measurements. Biochem J. 1959 Mar;71(3):544–551. doi: 10.1042/bj0710544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CACCIOLA E., CRISTALDI R., GIUSTOLISI R. [Activity of the elastase type in the blood serum]. Boll Soc Ital Biol Sper. 1961 Dec 15;37:1181–1185. [PubMed] [Google Scholar]
- CHAO S., SCIARRA J. J., VOSBURGH G. J. A spectrophotometric method for determination of elastase and serum elastase inhibitor. Proc Soc Exp Biol Med. 1962 Feb;109:342–345. doi: 10.3181/00379727-109-27196. [DOI] [PubMed] [Google Scholar]
- FINDLAY G. H. On elastase and the elastic dystrophies of the skin. Br J Dermatol. 1954 Jan;66(1):16–24. doi: 10.1111/j.1365-2133.1954.tb12553.x. [DOI] [PubMed] [Google Scholar]
- GORE I., LARKEY B. J. Functional activity of aortic mucopolysaccharides. J Lab Clin Med. 1960 Dec;56:839–846. [PubMed] [Google Scholar]
- HALL D. A., CZERKAWSKI J. W. The purification of the proteolytic component of elastase. Biochem J. 1959 Oct;73:356–361. doi: 10.1042/bj0730356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL D. A., CZERKAWSKI J. W. The reaction between elastase and elastic tissue. 4. Soluble elastins. Biochem J. 1961 Jul;80:121–128. doi: 10.1042/bj0800121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL D. A. The complex nature of the enzyme elastase. Arch Biochem Biophys. 1957 Apr;67(2):366–377. doi: 10.1016/0003-9861(57)90291-6. [DOI] [PubMed] [Google Scholar]
- HALL D. A. The reaction between elastase and elastic tissue. 1. The substrate. Biochem J. 1955 Mar;59(3):459–465. doi: 10.1042/bj0590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKAS E., FOLDES I., BANGA I. The elastase and trypsin contents of the pancreatic secretion in dogs. Acta Physiol Acad Sci Hung. 1951;2(3-4):333–341. [PubMed] [Google Scholar]
- LAMY F., LANSING A. I. Localization of proelastase in the goose-fish (Lophius piscatorius). Proc Soc Exp Biol Med. 1961 Jan;106:160–162. doi: 10.3181/00379727-106-26270. [DOI] [PubMed] [Google Scholar]
- LOEVEN W. A. THE ENZYMES OF THE ELASTASE COMPLEX. Int Rev Connect Tissue Res. 1963;1:183–240. doi: 10.1016/b978-1-4831-6755-8.50010-3. [DOI] [PubMed] [Google Scholar]
- MATHESON A. T., TIGANE E., HANES C. S. Quantitative chromatographic methods. 5. An improved ninhydrin-hydrindantin reagent. Can J Biochem Physiol. 1961 Feb;39:417–425. doi: 10.1139/o61-040. [DOI] [PubMed] [Google Scholar]
- MOON H. D., MCIVOR B. C. Elastase in the exocrine pancreas: localization with fluorescent antibody. J Immunol. 1960 Jul;85:78–80. [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F. Purification and specificity of pancreatic elastase. Biochem J. 1961 Jan;78:156–163. doi: 10.1042/bj0780156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OAKLEY C. L., BANERJEE N. G. Bacterial elastases. J Pathol Bacteriol. 1963 Apr;85:489–506. [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., GILFILLAN R. F., BARDAWIL W. A. A plate assay for elastase. Nature. 1960 Oct 22;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- SCIARRA J. J., CHAO S., MANDL I. Quantitative assay of elastase inhibitor in serum during pregnancy. Am J Obstet Gynecol. 1963 Jul 15;86:753–761. doi: 10.1016/s0002-9378(16)35191-2. [DOI] [PubMed] [Google Scholar]


