Abstract
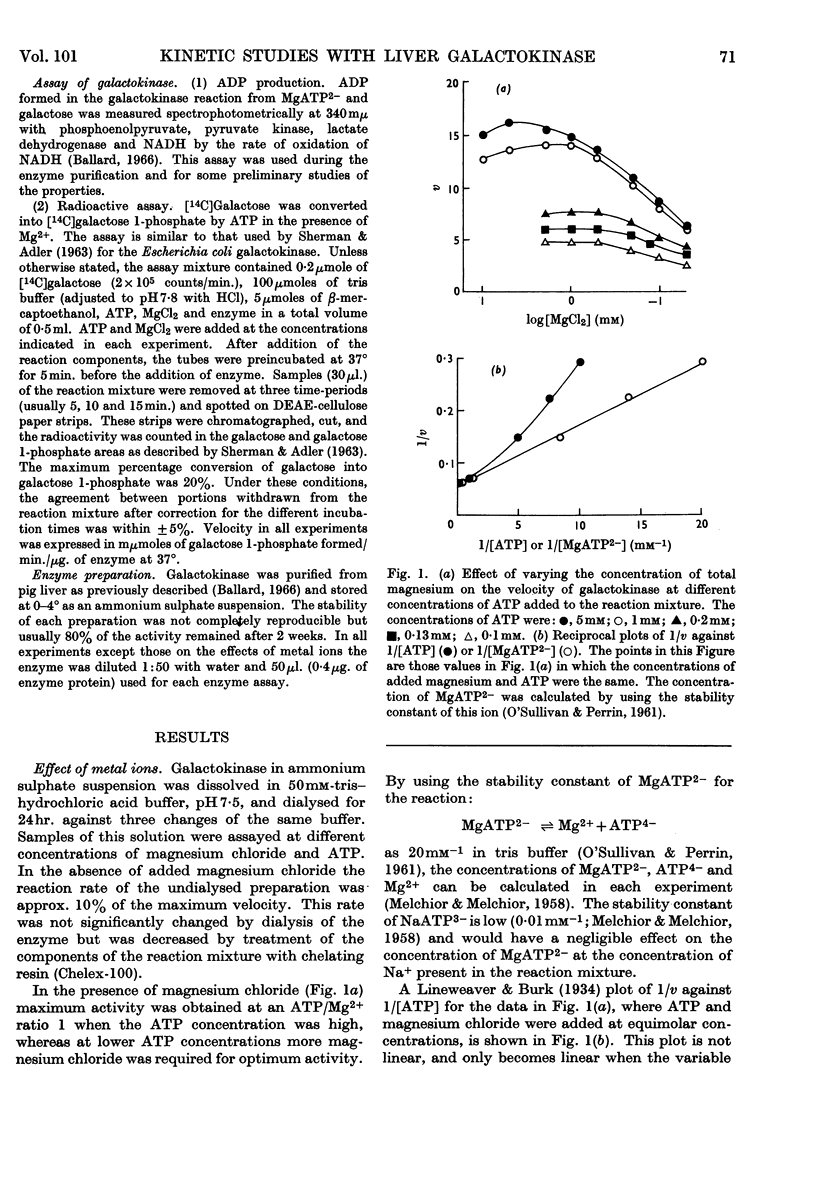
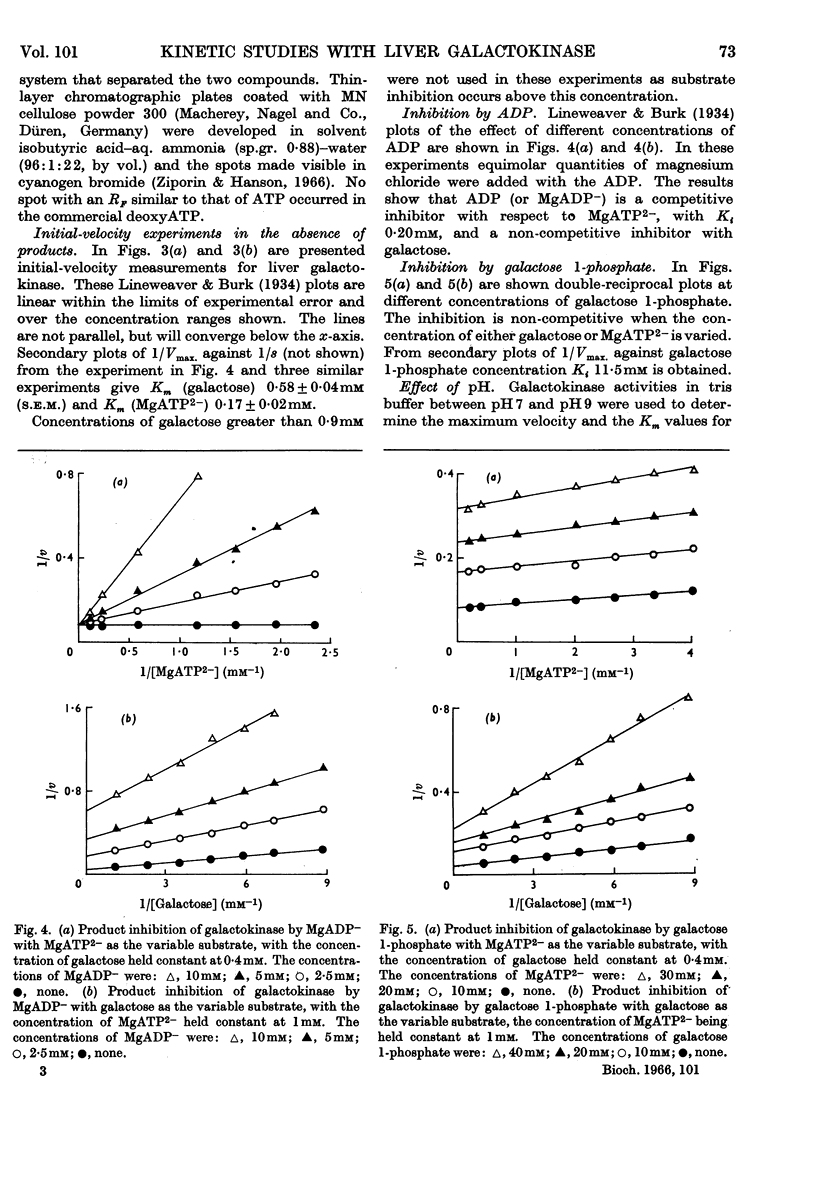
1. Kinetic measurements of the forward reaction catalysed by ATP–galactose phosphotransferase were carried out with a purified preparation from pig liver. 2. The rate of reaction at pH7·8 is dependent on the concentration of MgATP2− rather than total ATP or magnesium chloride concentrations. 3. The effect of changes in pH on Km (galactose), Km (MgATP2−) and Vmax. was studied. 4. Of several possible nucleotide substrates only ATP and deoxyATP were effective. 5. The initial-velocity patterns both in the absence and presence of products were determined. 6. Galactose 1-phosphate is a non-competitive inhibitor when either galactose or MgATP2− was the variable substrate. 7. MgADP− was a non-competitive inhibitor with galactose and a competitive inhibitor with MgATP2− as variable substrate. 8. These results are consistent with an ordered reaction pathway in which galactose combines with an initial enzyme–MgATP2− complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J. Purification and properties of galactokinase from pig liver. Biochem J. 1966 Jan;98(1):347–352. doi: 10.1042/bj0980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of yeast hexokinase. J Biol Chem. 1962 Oct;237:3027–3032. [PubMed] [Google Scholar]
- Gaffney T. J., O'Sullivan W. J. Kinetic studies of the activation of adenosine triphosphate-lombricine phosphotransferase by magnesium ions. Biochem J. 1964 Jan;90(1):177–181. doi: 10.1042/bj0900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson T. L., Fromm H. J. Rat skeletal muscle hexokinase. I. Kinetics and reaction mechanism. J Biol Chem. 1965 Nov;240(11):4133–4139. [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. III. Kinetic studies. J Biol Chem. 1954 Sep;210(1):65–82. [PubMed] [Google Scholar]
- MELCHIOR N. C., MELCHIOR J. B. The role of complex metal ions in the yeast hexokinase reaction. J Biol Chem. 1958 Apr;231(2):609–623. [PubMed] [Google Scholar]
- MORRISON J. F., GRIFFITHS D. E., ENNOR A. H. The purification and properties of arginine phosphokinase. Biochem J. 1957 Jan;65(1):143–153. doi: 10.1042/bj0650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON J. F., O'SULLIVAN W. J. KINETIC STUDIES OF THE REVERSE REACTION CATALYSED BY ADENOSINE TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE. THE INHIBITION BY MAGNESIUM IONS AND ADENOSINE DIPHOSPHATE. Biochem J. 1965 Jan;94:221–235. doi: 10.1042/bj0940221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F., James E. The mechanism of the reaction catalysed by adenosine triphosphate-creatine phosphotransferase. Biochem J. 1965 Oct;97(1):37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. The stability constants of MgATP -2 ion. Biochim Biophys Acta. 1961 Sep 30;52:612–614. doi: 10.1016/0006-3002(61)90431-0. [DOI] [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- SCHWARZ V., GOLBERG L., KOMROWER G. M., HOLZEL A. Some disturbances of erythrocyte metabolism in galactosaemia. Biochem J. 1956 Jan;62(1):34–40. doi: 10.1042/bj0620034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL H. L., KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. I. Further considerations of enzyme inhibition and analysis of enzyme activation. Enzymologia. 1952 Feb;15(4):187–198. [PubMed] [Google Scholar]
- SHERMAN J. R., ADLER J. Galactokinse from Escherichia coli. J Biol Chem. 1963 Mar;238:873–878. [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF BOVINE HEART AND RABBIT MUSCLE LACTATE DEHYDROGENASES. J Biol Chem. 1964 Nov;239:3901–3907. [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. II. Kinetic studies. J Biol Chem. 1957 Jul;227(1):313–328. [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]
- WRATTEN C. C., CLELAND W. W. PRODUCT INHIBITION STUDIES ON YEAST AND LIVER ALCOHOL DEHYDROGENASES. Biochemistry. 1963 Sep-Oct;2:935–941. doi: 10.1021/bi00905a007. [DOI] [PubMed] [Google Scholar]


