Abstract
1. By the implantation of wedges containing indol-3-ylacetic acid and sucrose into blocks of undifferentiated bean-callus tissue it has been possible to induce the formation of xylem and phloem cells. 2. The differentiation has been investigated cytologically and measured chemically. 3. The optimum concentrations of the nutrients in the wedge, which gave differentiation closely resembling the vascular development found in the stem of the intact plant, was 0·1mg. of indol-3-ylacetic acid/l. and 2% sucrose. 4. The ratios of the xylose/arabinose concentrations of the tissues increased in the differentiated callus tissue compared with those of the undifferentiated tissue. A similar increase has been found for the ratios determined for xylem tissue compared with those for cambium. 5. The lignin content of the differentiated tissue compared with the undifferentiated tissue was greater in both the callus and stem tissue. 6. Chemical analysis of lignin showed that in the differentiated callus tissue it consisted of sub-units based on p-hydroxybenzaldehyde and vanillin. This was compared with the lignin obtained from undifferentiated callus tissue and that obtained from the tissues of the intact stem. 7. The results of the investigation have been discussed with reference to the problems of cell growth and differentiation and related to the changing patterns of the ultrastructure of the cell during its development.
Full text
PDF
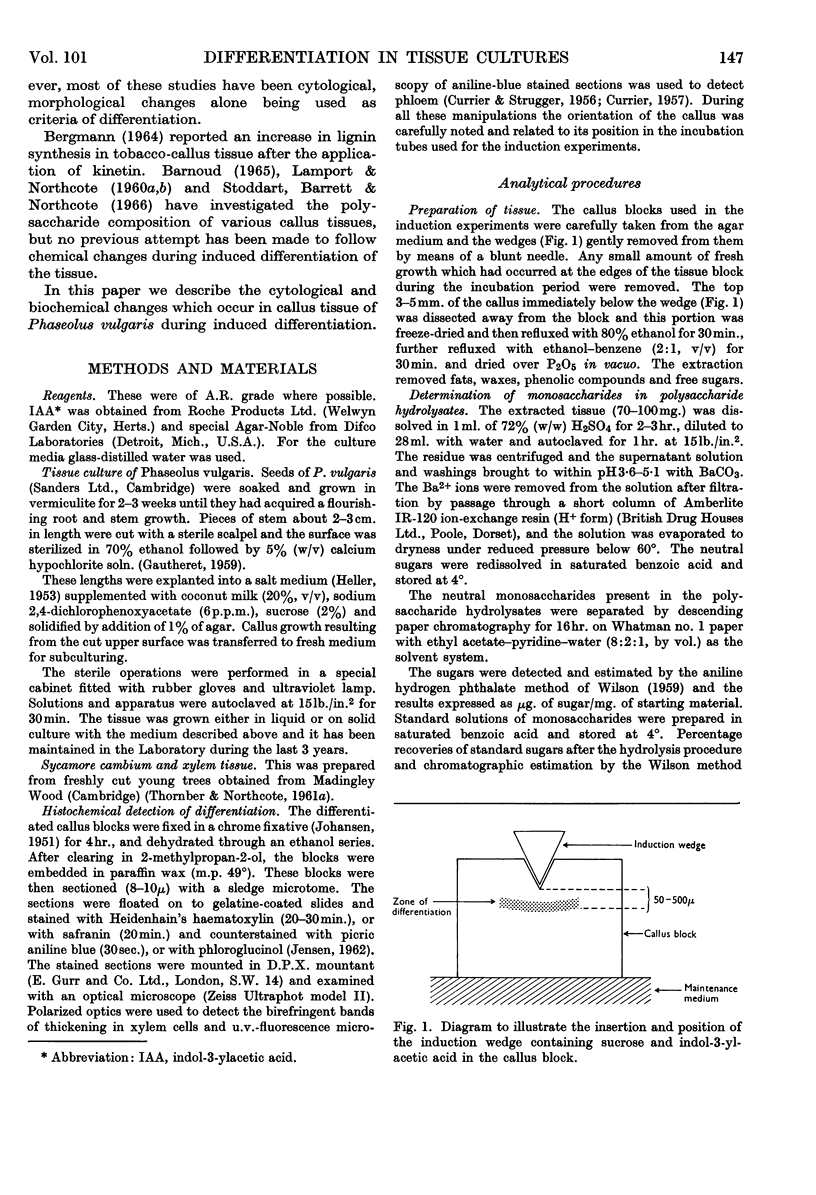

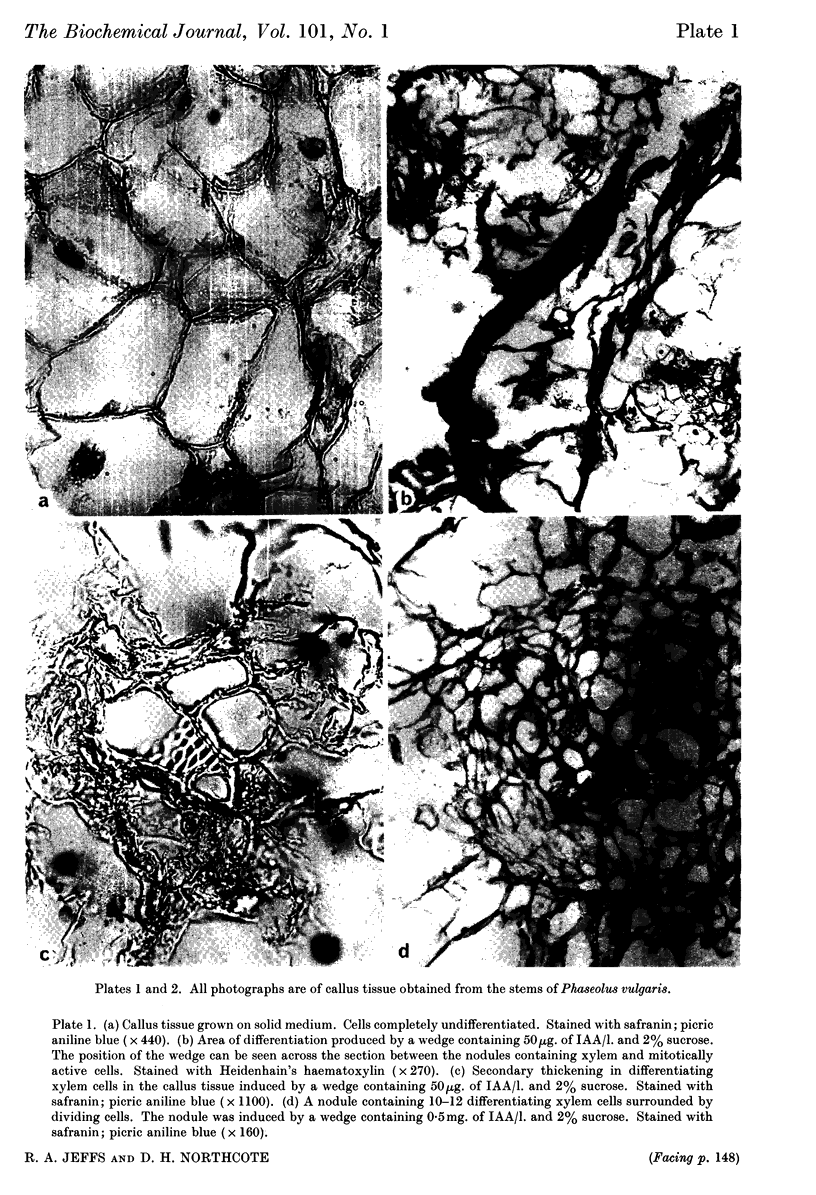
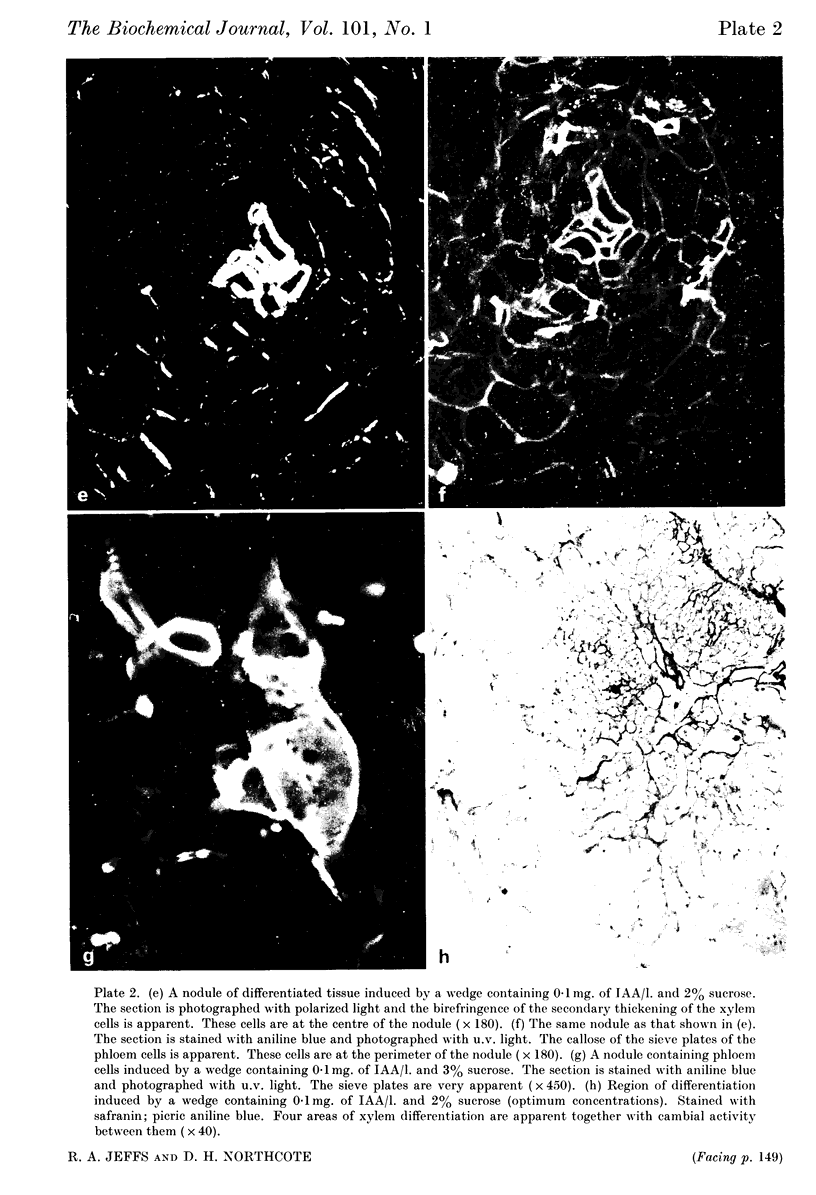
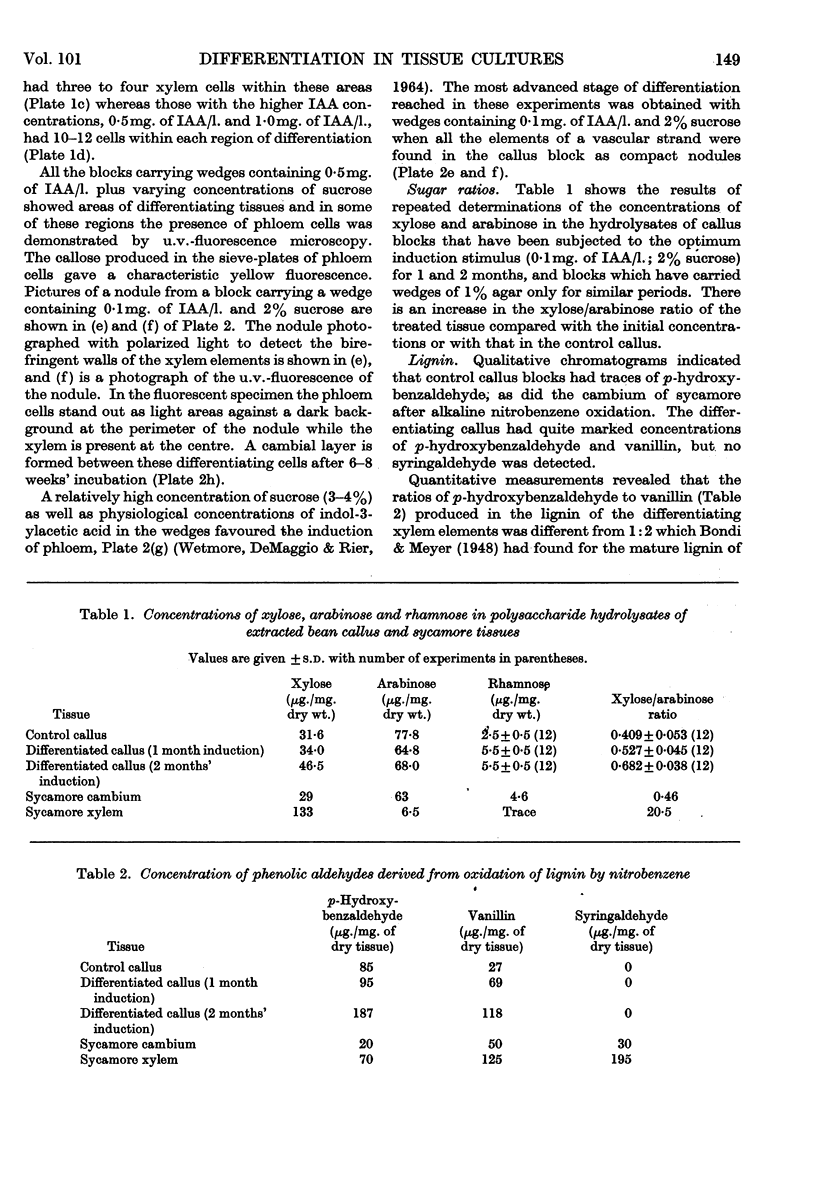
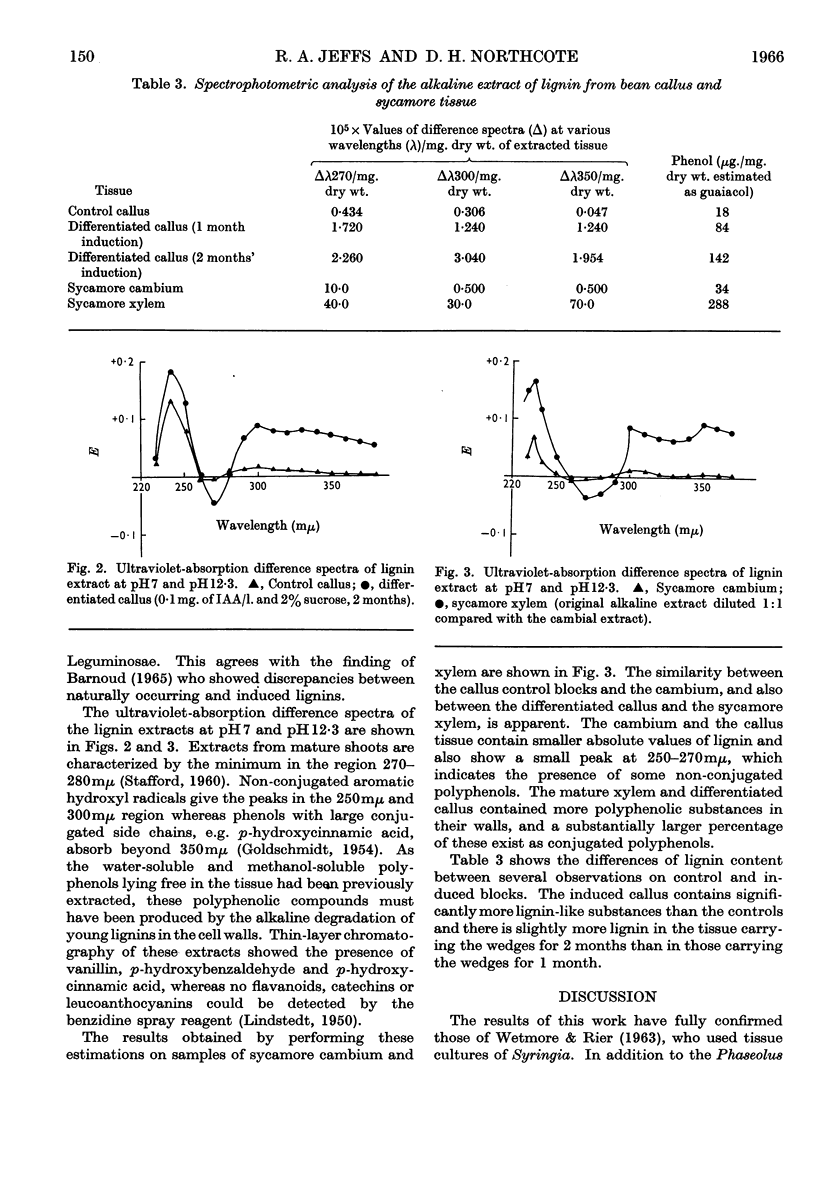


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRETT A. J., NORTHCOTE D. H. APPLE FRUIT PECTIC SUBSTANCES. Biochem J. 1965 Mar;94:617–627. doi: 10.1042/bj0940617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAND D. E. Separation of vanillin and syringaldehyde by paper partition chromatography. Nature. 1949 Dec 24;164(4182):1093–illust. doi: 10.1038/1641093a0. [DOI] [PubMed] [Google Scholar]
- Bondi A., Meyer H. Lignins in young plants. Biochem J. 1948;43(2):248–256. doi: 10.1042/bj0430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLUTTER M. E. Hormonal induction of vascular tissue in tobacco pith in vitro. Science. 1960 Aug 26;132(3426):548–549. doi: 10.1126/science.132.3426.548. [DOI] [PubMed] [Google Scholar]
- Earle E. D., Torrey J. G. Morphogenesis in cell colonies grown from Convolvulus cell suspensions plated on synthetic media. Am J Bot. 1965 Oct;52(9):891–899. [PubMed] [Google Scholar]
- Esau K., Cheadle V. I. AN EVALUATION OF STUDIES ON ULTRASTRUCTURE OF SIEVE PLATES. Proc Natl Acad Sci U S A. 1961 Nov;47(11):1716–1726. doi: 10.1073/pnas.47.11.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid W. Z., Neufeld E. F., Feingold D. S. SUGAR NUCLEOTIDES IN THE INTERCONVERSION OF CARBOHYDRATES IN HIGHER PLANTS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):905–915. doi: 10.1073/pnas.45.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. A., Ashton M. Composition of Developing Primary Wall in Onion Root Tip Cells. I. Quantitative Analyses. Plant Physiol. 1960 May;35(3):313–323. doi: 10.1104/pp.35.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Mollenhauer H. H., Whaley W. G. Ultrastructural changes in the root apex. Symp Soc Exp Biol. 1963;17:74–84. [PubMed] [Google Scholar]
- NORTHCOTE D. H. THE BIOLOGY AND CHEMISTRY OF THE CELL WALLS OF HIGHER PLANTS, ALGAE, AND FUNGI. Int Rev Cytol. 1963;14:223–265. doi: 10.1016/s0074-7696(08)60024-8. [DOI] [PubMed] [Google Scholar]
- Northcote D. H. Changes in the cell walls of plants during differentiation. Symp Soc Exp Biol. 1963;17:157–174. [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., Northcote D. H. Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci. 1966 Mar;1(1):109–120. doi: 10.1242/jcs.1.1.109. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Stafford H. A. Differences Between Lignin-like Polymers Formed by Peroxidation of Eugenol and Ferulic Acid in Leaf Sections of Phleum. Plant Physiol. 1960 Jan;35(1):108–114. doi: 10.1104/pp.35.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. 3. Xylan, glucomannan and alpha-cellulose fractions. Biochem J. 1962 Feb;82:340–346. doi: 10.1042/bj0820340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. I. Main components. Biochem J. 1961 Dec;81:449–455. doi: 10.1042/bj0810449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil V., Hildebrandt A. C. Differentiation of Tobacco Plants from Single, Isolated Cells in Microcultures. Science. 1965 Nov 12;150(3698):889–892. doi: 10.1126/science.150.3698.889. [DOI] [PubMed] [Google Scholar]
- WOODING F. B., NORTHCOTE D. H. THE DEVELOPMENT OF THE SECONDARY WALL OF THE XYLEM IN ACER PSEUDOPLATANUS. J Cell Biol. 1964 Nov;23:327–337. doi: 10.1083/jcb.23.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]