Abstract
The antifungal efficacy, pharmacokinetics, and safety of caspofungin (CAS) were investigated in the treatment and prophylaxis of invasive pulmonary aspergillosis due to Aspergillus fumigatus in persistently neutropenic rabbits. Antifungal therapy consisted of 1, 3, or 6 mg of CAS/kg of body weight/day (CAS1, CAS3, and CAS6, respectively) or 1 mg of deoxycholate amphotericin B (AMB)/kg/day intravenously for 12 days starting 24 h after endotracheal inoculation. Prophylaxis (CAS1) was initiated 4 days before endotracheal inoculation. Rabbits treated with CAS had significant improvement in survival and reduction in organism-mediated pulmonary injury (OMPI) measured by pulmonary infarct score and total lung weight (P < 0.01). However, animals treated with CAS demonstrated a paradoxical trend toward increased residual fungal burden (log CFU per gram) and increased serum galactomannan antigen index (GMI) despite improved survival. Rabbits receiving prophylactic CAS1 also showed significant improvement in survival and reduction in OMPI (P < 0.01), but there was no effect on residual fungal burden. In vitro tetrazolium salt hyphal damage assays and histologic studies demonstrated that CAS had concentration- and dose-dependent effects on hyphal structural integrity. In parallel with a decline in GMI, AMB significantly reduced the pulmonary tissue burden of A. fumigatus (P ≤ 0.01). The CAS1, CAS3, and CAS6 dose regimens demonstrated dose-proportional exposure and maintained drug levels in plasma above the MIC for the entire 24-h dosing interval at doses that were ≥3 mg/kg/day. As serial galactomannan antigen levels may be used for therapeutic monitoring, one should be aware that profoundly neutropenic patients receiving echinocandins for aspergillosis might have persistent galactomannan antigenemia despite clinical improvement. CAS improved survival, reduced pulmonary injury, and caused dose-dependent hyphal damage but with no reduction in residual fungal burden or galactomannan antigenemia in persistently neutropenic rabbits with invasive pulmonary aspergillosis.
Invasive pulmonary aspergillosis is an important cause of morbidity and mortality in patients receiving cytotoxic chemotherapy for leukemia, bone marrow transplantation, and aplastic anemia (3, 12, 15, 28, 44, 45). Amphotericin B (AMB) is the most widely used drug for treating invasive pulmonary aspergillosis; however, its use is limited by dose-dependent nephrotoxicity (20, 53). Lipid formulations of AMB are significantly less nephrotoxic with similar therapeutic efficacy; however, their use is limited by cost and infusion-related toxicity (19). Itraconazole has also been used for the treatment of invasive aspergillosis in neutropenic patients, but its oral formulations are either erratically absorbed or not well tolerated (29). Little is known about the recently introduced parenteral formulation of itraconazole. All of these compounds directly or indirectly target the fungal cell membrane.
The use of echinocandins provides a novel approach to targeting Aspergillus by inhibiting the synthesis of 1,3-β-d-glucan (13, 16, 21, 22, 34, 55). 1,3-β-d-Glucan is important for osmotic stability of fungal elements and plays an important role in cell growth and replication. By targeting this site, the cell wall of Aspergillus spp. may be disrupted and cell death can eventually occur (35).
In vitro studies have demonstrated the potent antifungal activity of caspofungin (CAS) against Candida spp. (5, 6, 32, 37, 39, 42, 43, 50). Additional studies have demonstrated that CAS is active against clinical isolates of filamentous fungi such as Aspergillus spp. and other molds (4, 6, 14, 46). CAS also has been evaluated in animal models of disseminated candidiasis (1, 2, 24, 25), disseminated aspergillosis (1, 2), cryptococcosis (1), histoplasmosis (23), and Pneumocystis carinii pneumonia (47).
CAS was recently approved for the treatment of invasive aspergillosis in patients refractory to or intolerant of conventional therapy. However, the database of treatment of invasive pulmonary aspergillosis in persistently neutropenic patients with CAS is limited.
Little is known about the use of CAS in the treatment of pulmonary aspergillosis in persistently neutropenic hosts. We therefore investigated the antifungal efficacy, galactomannan antigenemia, drug disposition, and safety of CAS in experimental pulmonary aspergillosis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Organism.
Aspergillus fumigatus strain 4215 (NIH 4215, ATCC MYA-1163), which was obtained from a fatal case of pulmonary aspergillosis, was used in all experiments. The organism was subcultured from a frozen isolate (stored at −70°C) onto Sabouraud dextrose slants (BBL, Cockeysville, Md.) and incubated for 24 h at 37°C. The slants were then allowed to grow at room temperature for an additional 5 days before conidia were harvested.
MICs were determined according to NCCLS tentative standard M27-A microdilution methods (17, 18, 40). The inoculum of A. fumigatus was prepared in normal saline (NS) to an optical density (OD) of 80 to 82% to give a conidium concentration of 1.0 × 106 to 5.0 × 106 CFU/ml in sterile NS. Aliquots of 0.1 ml of serially diluted drug and 0.1 ml of the inoculum suspension were inoculated into microtiter wells and incubated for 48 h at 35°C. The final concentrations of CAS (Merck & Co., Inc., Rahway, N.J.) ranged from 0.02 to 128 μg/ml. MICs were also determined by substituting antibiotic medium 3 (AM-3) (NIH Media Unit, Bethesda, Md.) for RPMI 1640 with phenol red (BioWhittaker, Walkerville, Md.). The MIC was defined as the lowest concentration of a drug that allowed no (0) or minimal (1+) growth on a scale ranging from 0 to 4+. The minimal fungicidal concentration (MFC) was then determined by plating 100 μl from those wells that showed no turbidity. The lowest concentration of drug that grew fewer than 3 colonies was designated as the MFC. The MICs of CAS and AMB were 0.06 and 1.0 μg/ml, respectively, in RPMI and 0.06 and 0.5 μg/ml, respectively, in AM-3. The MFC of CAS was 128 μg/ml in AM-3 and >128 μg/ml in RPMI. The MFC of AMB was 1.0 μg/ml in AM-3 and 1.0 μg/ml in RPMI.
MTT hyphal damage assays.
A rapid colorimetric assay with tetrazolium salt (MTT) (36) was conducted to evaluate the effect of echinocandin on damage to the hyphae of A. fumigatus. The yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, Mo.) is cleaved by dehydrogenases of metabolically active fungi, reducing it to its purple MTT formazan derivative, which is then quantitated by spectrophotometry after alcohol extraction. Fungal viability was evaluated at 1-, 3-, and 6-h time points at concentrations of CAS ranging from 0.01 to 10.0 μg/ml and at a 0.5-μg/ml concentration of AMB; results were reported as the mean percent damages for each concentration. Absorbance (A) readings were made on a microplate spectrophotometer (Multiscan MMC/340; Titertek, Huntsville, Ala.) at 570 and 690 nm. Hyphal damage was reported as a percentage of absorption of treated cells compared to untreated control cells by using the following equation: percent kill = 1 − (A570–690 with drug/A570–690 without drug) × 100.
Animals.
Healthy female New Zealand White rabbits (Hazleton Research Products, Inc., Denver, Pa.) weighing 2.7 to 3.8 kg at the time of inoculation were used in all experiments. These studies were approved by the Animal Care and Use Committee of the National Cancer Institute. All rabbits were housed and monitored under humane care and use in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and according to National Institutes of Health guidelines for animal care and in fulfillment of the guidelines of the National Research Council (41). Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum. A total of 77 rabbits were used for all experiments. Vascular access was established in each rabbit by the surgical placement of a Silastic tunneled central venous catheter as previously described (56). The Silastic catheter permitted nontraumatic venous access for repeated blood sampling for the study of biochemical and hematological parameters, pharmacokinetics in plasma, serum galactomannan, and administration of parenteral agents. Serum samples were drawn from all rabbits at the initiation of immunosuppression, during the course of pulmonary aspergillosis, and before death. Rabbits were euthanatized by intravenous (i.v.) administration of sodium pentobarbital (65 mg [1 ml]/kg of body weight; The Butler Company, Columbus, Ohio) at the end of each experiment, 24 h after administration of the last dose of study drug.
Inoculation.
Pulmonary aspergillosis was established as previously described (19). For each experiment, the inoculum of A. fumigatus was prepared from Sabouraud dextrose slants inoculated as described above. Conidia were harvested under a laminar airflow hood with a solution of 10 ml of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in 0.9% NaCl (Quality Biological, Inc., Gaithersburg, Md.), transferred to a 50-ml conical tube, washed, and counted with a hemacytometer. The concentration was adjusted in order to give each rabbit a predetermined inoculum of 108 conidia of A. fumigatus in a volume of 250 to 350 μl. The concentrations of the inocula were confirmed by serial dilutions cultured on Sabouraud glucose agar (SGA).
Inoculation was performed on day 2 of the experiments while the animals were under general anesthesia. Each rabbit was anesthetized with 0.5 to 1.0 ml of a 2:1 mixture (vol/vol) of i.v. administered ketamine (100 mg/ml) (Ketaset; Phoenix Scientific, Inc., St. Joseph, Mo.) and xylazine (20 mg/ml) (Rompun; Bayer Corp., Agriculture Division, Animal Health, Shawnee Mission, Kans.). Once satisfactory anesthesia was obtained, a Flagg O straight-blade laryngoscope (Welch-Allyn, Inc., Skaneateles Falls, N.Y.) was inserted in the oral cavity until the vocal cords were clearly visible. The A. fumigatus inoculum was then administered intratracheally with a tuberculin syringe attached to a 5 1/4-in. 16-gauge Teflon catheter (Becton Dickinson Infusion Therapy Systems, Inc., Sandy, Utah).
Immunosuppression and maintenance of neutropenia.
To simulate the conditions of persistent neutropenia, treatment with cytarabine (Ara-C) (Cytosar-U; The Upjohn Company, Kalamazoo, Mich.) was initiated i.v. 1 day before the endotracheal inoculation of the animals. Profound and persistent neutropenia (a granulocyte concentration of <100 granulocytes/μl) was achieved by an initial course of 525 mg of Ara-C per m2 for five consecutive days. A maintenance dose of 484 mg of Ara-C per m2 was administered for four additional days on days 8, 9, 13, and 14 of the experiment to maintain persistent neutropenia. Concomitant thrombocytopenia was present in a range of 30,000 to 50,000 platelets/μl. Methylprednisolone (Abbott Laboratories, North Chicago, Il.) at a dose of 5 mg/kg of body weight/day was administered on days 1 and 2 of the experiment to inhibit macrophage activity against conidia in order to facilitate establishment of infection.
Ceftazidime (75 mg/kg given i.v. twice daily; Glaxo, Inc., Research Triangle Park, N.C.), gentamicin (5 mg/kg given i.v. every other day; Elkins-Sinn, Inc., Cherry Hill, N.J.), and vancomycin (15 mg/kg given i.v. daily; Abbott Laboratories) were administered from day 4 of immunosuppression until study completion to prevent opportunistic bacterial infections during neutropenia. In order to prevent antibiotic-associated diarrhea due to Clostridium spiroforme, all rabbits continuously received 50 mg of vancomycin per liter of drinking water.
Total leukocyte counts and the percentages of granulocytes were monitored twice weekly with a Coulter counter (Coulter Corporation, Miami, Fla.) and by peripheral blood smears and differential counts, respectively.
Antifungal compounds and treatment regimens.
Rabbits were grouped to receive CAS or AMB deoxycholate (Bristol-Myers Squibb Company, Princeton, N.J.) for treatment of established invasive pulmonary aspergillosis or no drug (untreated controls). CAS was provided as a lyophilized powder and dissolved according to the recommendations of the manufacturer in sterile water to produce a 50-mg/ml stock solution that was maintained at −70°C. Prior to use, CAS was freshly diluted with sterile water to 2-, 5-, and 10-mg/ml solutions for the 1-, 3-, and 6-mg/kg/day dosage groups (CAS1, CAS3, and CAS6, respectively). The reconstituted CAS was administered at ambient temperature as a slow i.v. bolus over 1 min. AMB was diluted with sterile water to a 1-mg/ml concentration and slowly administered i.v. at a rate of 1 mg/kg/day (0.1 ml every 10 s). Antifungal therapy was initiated 24 h after endotracheal inoculation. CAS and AMB therapies were continued throughout the course of the experiments for a maximum of 12 days in surviving rabbits.
Prophylactic regimen.
In order to investigate the efficacy of CAS for prevention of pulmonary aspergillosis in a persistently neutropenic rabbit model, we compared CAS-treated rabbits to untreated controls. The prophylaxis studies used the same methods as described above with the following exceptions. Based upon assessment of response in the therapeutic model, a single dose regimen of CAS was selected for prophylaxis. Rabbits received CAS1 for 4 days before endotracheal inoculation. Administration of CAS1 was then continued for a maximum of 12 days. In order to simulate the low initial tissue burden of A. fumigatus in the setting of antifungal prophylaxis, the administered inoculum was 5 × 107 conidia. All other methods, including outcome variables, were identical for both the treatment and prophylaxis experiments.
Outcome variables.
The following panel of outcome variables was used to assess antifungal efficacy: survival, pulmonary infarct score, lung weight, residual fungal burden (log CFU/gram), fungal growth (log CFU/gram) in bronchoalveolar lavage (BAL) fluid, computerized tomography (CT) scores, galactomannan index (GMI), and histopathology. Pulmonary infarct score, lung weight, and CT scan were measures of organism-mediated pulmonary injury.
Survival.
The survival time in days postinoculation was recorded for each rabbit in each group. Surviving rabbits were euthanatized by sodium pentobarbital anesthesia on day 13 postinoculation, 24 h after the last dose of study drug.
Pulmonary lesion scores.
The entire heart-lung block was carefully resected at autopsy. The heart was then dissected away from the lungs, leaving the tracheobronchial tree and lungs intact. The lungs were weighed and inspected by at least two observers who were blinded to the treatment group, and the observers recorded the hemorrhagic infarct lesions (if any) in each individual lobe. Hemorrhagic infarcts were dark red consolidated lesions that corresponded histologically to coagulative necrosis and intra-alveolar hemorrhage. The number of positive lobes was added together, and the mean value of all positive lobes was calculated for each treatment group.
BAL.
BAL was performed on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile NS into the clamped trachea with a sterile 12-ml syringe. This process was repeated for a total infusion of 20 ml of NS. The lavage material was then centrifuged for 10 min at 500 × g. The supernatant was discarded, leaving 2 ml of fluid in which the pellet was then resuspended. A 0.1-ml sample of this fluid and 0.1 ml of dilution (10−1) of this fluid were cultured on 5% SGA plates.
Histopathology.
Pulmonary lesions were excised and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were then sectioned and stained with either periodic acid-Schiff or Grocott-Gomori methenamine-silver stain. Tissues were microscopically examined for pulmonary injury and structural changes in Aspergillus hyphae.
Fungal cultures.
Lung tissue from each rabbit was sampled and cultured by a standard excision of tissue from each lobe. Each fragment was weighed individually, placed in a sterile polyethylene bag (Tekmar Corp., Cincinnati, Ohio), and homogenized with sterile saline for 30 s per tissue sample (Stomacher 80; Tekmar) (52). Lung homogenate dilutions (10−1 and 10−2) were prepared in sterile saline. Aliquots (100 μl) from homogenates and homogenate dilutions were plated onto SGA plates and incubated at 37°C for the first 24 h and then left at room temperature for another 24 h. The number of CFU of A. fumigatus was counted and recorded for each lobe, and the number of CFU per gram was calculated. A finding of one colony of A. fumigatus was considered positive.
CT.
Serial CT of the lungs was performed during all experiments in order to monitor the effects of antifungal therapy on organism-mediated pulmonary injury during the course of infection. Briefly, rabbits were sedated with ketamine and xylazine and then placed prone, head first, on the scanning couch. CT was performed with the ultrafast electron beam CT scanner (model C-100XL; Imatron, Oyster Point, Calif.), as previously described (54). Using the high-resolution, table-incremented, volume acquisition mode, 3-mm-thick ultrafast CT scans were performed every 4 s. A small scan circle and a 9-cm-diameter reconstruction circle with a matrix of 512 by 512 were used, which resulted in a pixel size of less than 1 mm. Scan parameters were 130 kV and 630 mA, and scan duration was 100 ms. In virtually all cases, 30 slices were sufficient to scan the entire thorax of the rabbit. Images were photographed using lung windows with a level of −600 Hounsfield units and a width of 1,800 Hounsfield units. The radiologic features of invasive pulmonary aspergillosis observed in this experimental system are similar to those reported in persistently neutropenic patients (9, 10, 31, 33).
Galactomannan assay.
Blood was collected every other day from each rabbit for the determination of serum galactomannan concentrations. Serum galactomannan concentrations were determined by the Platelia Aspergillus EIA (Genetic Systems/Sanofi Diagnostic Pasteur, Redmond, Wash.) one-stage immunoenzymatic sandwich microplate assay method, which was performed according to the manufacturer’s directions (Platelia Aspergillus 62797, Immunoenzymatic detection of galactomannan antigen of Aspergillus in serum, Sanofi Diagnostic Pasteur). The assay uses the rat monoclonal antibody EB-A2, which is directed against Aspergillus galactomannan (49). The monoclonal antibody is used to sensitize the wells of the microplate and to bind the antigen. Peroxidase-linked monoclonal rat antibody is used as the detector antibody. The optical absorbances of specimens and controls were determined by a microplate spectrophotometer equipped with 450- and 620-nm filters (Titertek Multiscan MMC/340).
Enzyme immunoassay data were expressed as a serum GMI plotted over time. The GMI for each test serum is equal to the OD of a sample divided by the OD of a threshold serum provided in the test kit. Sera with GMIs of less than 1 were considered negative. Sera with GMIs of greater than 1.5 were considered positive. Sera with GMIs between 1 and 1.5 were considered indeterminate. Serial serum galactomannan levels were plotted over time as a function of antifungal compound and time of initiation of study drug treatment (treatment versus prophylaxis).
Pharmacokinetic studies.
The plasma pharmacokinetics of CAS were investigated in 12 infected animals per dosage cohort by using optimal plasma sampling. Time points for optimal plasma sampling were determined on the basis of full plasma concentration profiles obtained in healthy rabbits (26) with the aid of the Adapt II computer program (D. Z. D’Argenio and A. Schumitzky, Adapt II user’s guide, Biomedical Simulations Resource, University of Southern California, Los Angeles). Plasma sampling was performed on day 5 of antifungal therapy. Blood samples were drawn at 0.17, 2.5, 8, 12, and 24 h postdose. Similarly, infected lung tissue and BAL fluid were sampled postmortem as described above for the analysis of CAS effectiveness.
Blood samples were collected in heparinized syringes. Plasma was immediately separated by centrifugation. Plasma, lung tissue, and BAL fluid were stored at −80°C until shipment in dry ice to the laboratories of Merck Sharp & Dohme-Chibret, Riom, France, for assay.
Drug levels were determined after solid-phase extraction and dilution in the mobile phase by reversed-phase high-performance liquid chromatography. Lung tissues were homogenized in sterile NS prior to extraction. The mobile phase consisted of acetonitrile-0.01 M KH2PO4 (60:40 [vol/vol]), pH 3. Separation was achieved using a Brownlee Cyano column (220- by 4.6-mm inside diameter, 5-μm particle size; Perkin Elmer, Norwalk, Conn.). CAS was detected by fluorometric detection (excitation, 224 nm; emission, 302 nm ). Quantitation was based on an internal standard method using the semisynthetic echinocandin L-733,560 as the internal standard. Eight-point standard curves (range of concentrations, 0.15 to 10 μg/ml) were linear with r2 values of greater than 0.998. The lower limits of quantitation were 0.15 μg/ml for plasma, 0.10 μg/ml for BAL material, and 0.17 μg/g for lung tissues. Accuracies were within ±15% and intra- and interday variability (precision) was <5%.
Pharmacokinetic parameters for CAS were determined by compartmental analysis and the Bayesian approach by using microconstants derived from pharmacokinetic studies in healthy rabbits (26) as priors. Pharmacokinetic modeling was performed with the Adapt II (D’Argenio and Schumitzky, Adapt II user’s guide) computer program using iterative weighted nonlinear least squares regression analysis. Model selection was guided by Akaike’s information criterion (57). Concentration-time profiles of CAS were best described by a 3-compartment open model with intravenous bolus input and linear first-order elimination from the central compartment (26). The model fit the optimal sampling-derived data well. The regression lines through the plot of observed versus estimated concentrations did not differ from the line of identity, and no bias was observed. r2 values for the individual fits ranged from 0.949 to 1.00 (mean, 0.997). Peak concentrations in plasma (Cmax) were determined as model-estimated concentrations immediately after bolus administration, and the values for the area under the concentration-time curve from 0 to 24 h (AUC0–24) were calculated from estimated plasma concentration profiles using the trapezoidal rule.
Toxicity studies.
Blood was collected from each rabbit every other day, starting the first day after inoculation and continuing throughout the treatment. Plasma samples were stored in Sarstedt tubes (Sarstedt, Inc., Newton, N.C.) at −70°C until all samples were processed simultaneously. Chemical determinations of potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, urea nitrogen, and total bilirubin concentrations in serum were performed on the penultimate sample drawn from each rabbit.
Statistical analysis.
Comparisons between groups were performed by analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons or the Mann-Whitney U test, as appropriate, against untreated controls. Kaplan-Meier survival plots were analyzed by the Mantel-Haenzsel chi-square test. All P values were two sided, and a P value of <0.05 was considered to be significant. Values are expressed as means ± standard errors of the means (SEMs).
RESULTS
MTT hyphal damage assays.
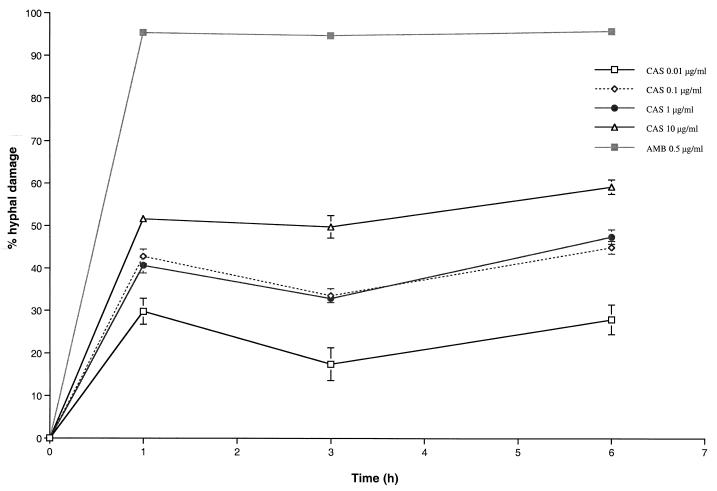
In vitro MTT hyphal damage assays demonstrated that there were significant (P < 0.0001) concentration-dependent effects. After 1 h, a 0.01-μg/ml concentration of CAS produced a mean percentage of hyphal damage of 28.12% ± 3.96%, whereas a 10.00-μg/ml concentration of CAS produced a mean percentage of hyphal damage of 51.59% ± 1.37% (Fig. 1). In comparison, a 0.5-μg/ml concentration of AMB produced a mean percentage of hyphal damage of 95.32% ± 0.41%. There was no significant time-dependent effect on hyphal damage for the further incubation for 24 h.
FIG. 1.
Hyphal damage to A. fumigatus by CAS versus AMB by MTT assay. In vitro MTT assays demonstrated significant (P < 0.0001 by ANOVA) concentration-dependent effects on hyphal damage. Values are given as means ± SEMs (error bars). As the SEMs were small for several time points, the error bars may not always be apparent in the hyphal damage curves.
Antifungal therapy.
There was a significant improvement in the survival of rabbits treated with CAS1 (3 of 12 [25%], P < 0.001), CAS3 (2 of 12 [16.7%], P < 0.01), and CAS6 (3 of 12 [25%], P < 0.05) in comparison with those of the untreated controls (Table 1). Survival was also improved in the overall population of CAS-treated rabbits, with 8 of 36 (22.2%) animals surviving through the entire duration of the study (P < 0.001).
TABLE 1.
Survival of persistently neutropenic rabbits with primary pulmonary aspergillosis treated with different doses of CAS or AMB compared to that of untreated control rabbits
| Treatment group (n) | Survival (days)
|
P valueb | |||
|---|---|---|---|---|---|
| Mean ± SEM | Median | Range | 95% CIa | ||
| Control (12) | 6.92 ± 0.69 | 6.0 | 4–13 | 5.40–8.44 | |
| CAS (all doses) (36) | 10.03 ± 0.36 | 10.0 | 6–13 | 9.31–10.75 | <0.001 |
| CAS1 (12) | 10.42 ± 0.54 | 10.0 | 7–13 | 9.22–11.61 | <0.001 |
| CAS3 (12) | 10.17 ± 0.61 | 10.5 | 5–13 | 8.82–11.52 | <0.01 |
| CAS6 (12) | 9.50 ± 0.70 | 9.0 | 6–13 | 7.96–11.04 | <0.05 |
| AMB (6) | 8.83 ± 0.48 | 9.0 | 7–10 | 7.61–10.06 | >0.05 |
95% CI, 95% confidence interval.
P values comparing survival values for the treated group to those for the control group were calculated by using ANOVA with Bonferroni’s correction for multiple comparisons.
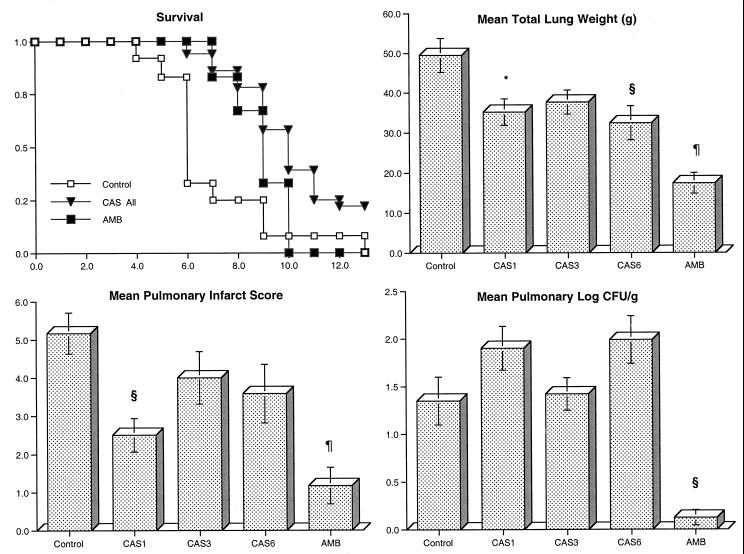
There also was a reduction in organism-mediated pulmonary injury as measured by total lung weight and pulmonary infarct score in rabbits treated with CAS and AMB (Fig. 2). The mean lung weights in rabbits treated with CAS1, CAS6, and AMB were significantly reduced compared to those of the untreated controls (P < 0.05, P < 0.01, and P < 0.001, respectively). No differences in lung weight were noted between the untreated controls and CAS3. CAS1- and AMB-treated animals showed a significant reduction in the mean pulmonary infarct score in comparison with untreated controls (P < 0.01 and P < 0.001, respectively). However, no differences in mean pulmonary infarct score were noted between untreated controls and animals treated with CAS3 and CAS6. As there were no dose-related effects of CAS on pulmonary injury (measured by pulmonary infarct scores or lung weights) over the dose range selected, all three dosage groups were combined for further analysis. There was a significant reduction of mean pulmonary infarct scores (3.36 ± 0.53 versus 5.17 ± 0.38, P = 0.0156) and mean lung weights (35.1 ± 4.27 g versus 49.5 ± 2.03 g, P = 0.0033) in CAS-treated rabbits in comparison with those of the untreated controls.
FIG. 2.
Response of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy measured by survival, mean lung weight, mean pulmonary infarct score, and mean pulmonary tissue concentration of residual organisms (log CFU/gram) in untreated controls (n = 12) and rabbits treated with CAS (CAS1 [n = 12], CAS3 [n = 12], CAS6 [n = 12]) or AMB (1 mg/kg/day) (n = 6). Values are given as means ± SEMs (error bars). P values are indicated as follows (in comparison to untreated controls, calculated by using ANOVA with Bonferroni’s correction for multiple comparisons): *, P < 0.05; §, P < 0.01; ¶, P < 0.001. For the measure of survival, the values on the x axis are days following inoculation and the values on the y axis are probability of survival.
CAS-treated animals demonstrated a paradoxical trend toward increased concentration of residual organisms (Fig. 2). By comparison, there was a significant quantitative reduction in A. fumigatus growth in pulmonary tissue from AMB-treated rabbits compared to that observed in the untreated controls (P < 0.01). There was a trend toward higher frequency of negative BAL cultures for A. fumigatus in rabbits treated with CAS than in untreated rabbits (Table 2), whereas BAL fluid from AMB-treated rabbits showed no detectable organisms in any sample.
TABLE 2.
BAL cultures for A. fumigatus in persistently neutropenic rabbits with pulmonary aspergillosis
| Treatment group (n) | Mean ± SEM (log CFU/g) | No. (%) of positive cultures |
|---|---|---|
| Control (12) | 0.79 ± 0.31 | 5 (41.6) |
| CAS (all doses) (36) | 0.6 ± 0.18 | 10 (27.8) |
| CAS1 (12) | 0.86 ± 0.32 | 5 (41.6) |
| CAS3 (12) | 0.53 ± 0.33 | 3 (25) |
| CAS6 (12) | 0.41 ± 0.27 | 2 (16.7) |
| AMB (6) | 0 | 0 |
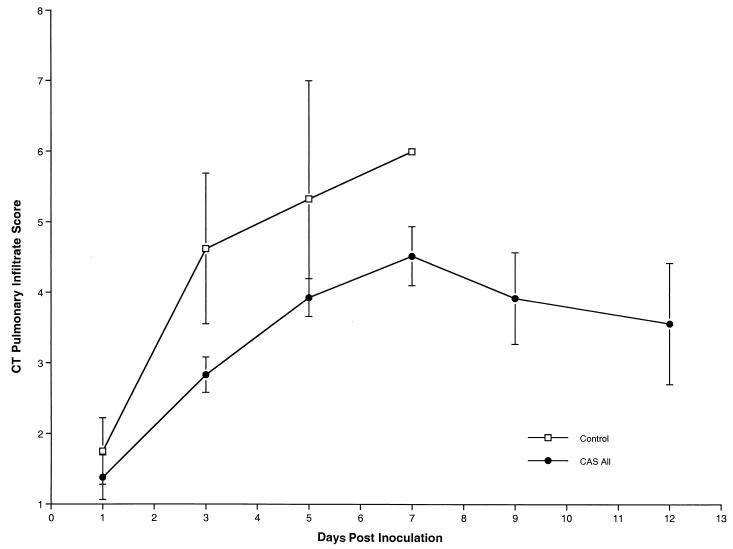
Consistent with the reduction in organism-mediated pulmonary injury, ultrafast CT scans demonstrated resolution of pulmonary infiltrates in rabbits treated with CAS (Fig. 3) (P < 0.0001 by ANOVA). As there was no significant intergroup difference of pulmonary infiltrate scores by CT scan among the different dosage cohorts of CAS, all three groups were combined for analysis. During the first 7 days of treatment, there was an increase in the number of pulmonary infiltrates. However, the magnitude of the pulmonary infiltrate scores in treated animals within the first 7 days was less than that of untreated controls. Following day 7 of treatment, there was a reduction in the number of infiltrates in rabbits receiving CAS therapy.
FIG. 3.
Pulmonary infiltrate scores determined by image analysis of serial CT scans of untreated control and CAS-treated rabbits from all groups. Animals treated with CAS demonstrated significant resolution of pulmonary infiltrates in comparison to untreated controls (P < 0.0001 by ANOVA). Pulmonary infiltrates increased during the first 7 days in untreated control and treated rabbits. The pulmonary infiltrate curve ends on day 7 due to mortality in untreated control rabbits. Pulmonary infiltrates declined following day 7 in the CAS treatment cohort.
Similarly there also was no dosage-related effect in the reduction of CT-measured pulmonary injury. When the results of CT monitoring are combined for all groups, there is a significant reduction in CT-measured pulmonary injury in CAS-treated rabbits.
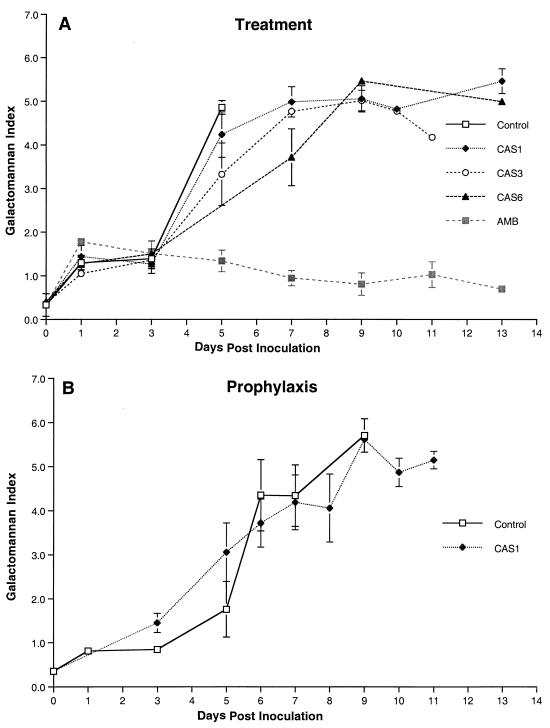
We also investigated the expression of galactomannan in the serum of rabbits with pulmonary aspergillosis as a surrogate marker of therapeutic response to the echinocandins (Fig. 4). The GMI was studied in animals receiving treatment (Fig. 4A) and prophylaxis (Fig. 4B). Serial serum samples in untreated control rabbits showed progressive galactomannan antigenemia correlating with progression of invasive pulmonary aspergillosis (Fig. 4A). Despite a reduction in pulmonary infiltrates, lung weights, and mortality, GMI increased in CAS-treated rabbits. This finding paralleled the previously noted paradoxical increase in the residual fungal burden (log CFU/gram) in the lung tissue of CAS-treated animals. A similar rise in GMI also was observed in rabbits receiving prophylaxis with CAS1 (Fig. 4B). Serial serum GMI values of AMB-treated rabbits were positive 1 day after inoculation and declined during the treatment. In parallel with a decline in GMI, AMB significantly reduced the pulmonary tissue burden of A. fumigatus (P ≤ 0.01) (Fig. 4A).
FIG. 4.
Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis in the treatment (A) and prophylaxis (B) groups.
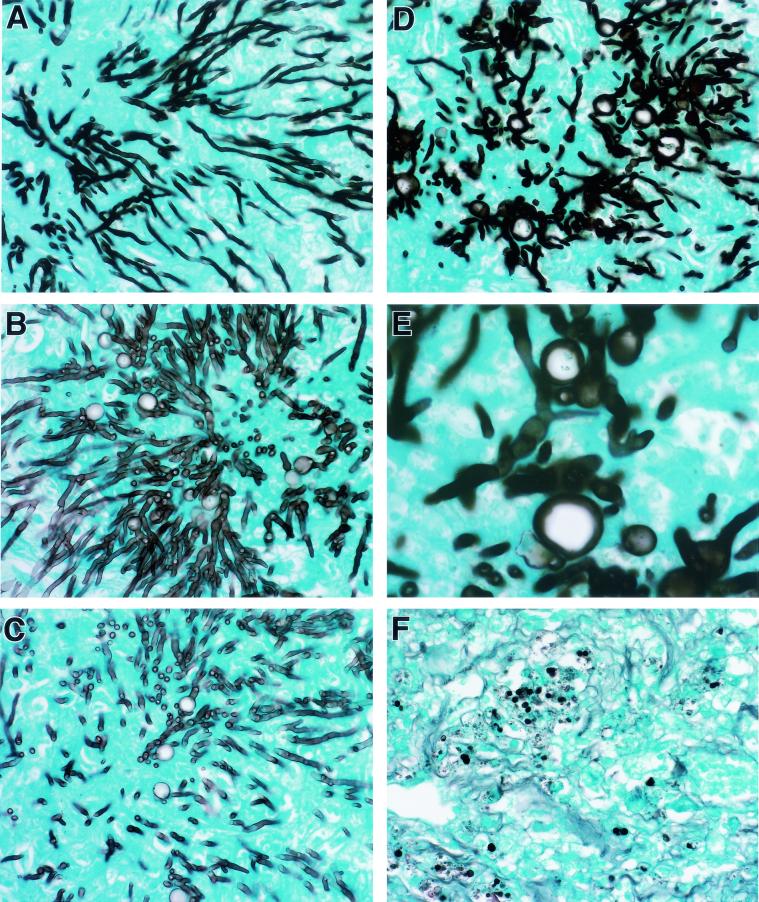
The histopathological features of aspergillosis were studied in the lungs of rabbits in all treatment groups. There was dose-dependent damage of hyphal structures in the lung tissue of CAS-treated rabbits. Each panel in Fig. 5 demonstrates a representative section of hyphal morphology in a corresponding treatment group. Organisms from untreated control rabbits demonstrated typical dichotomously branching septate hyphae of A. fumigatus (Fig. 5A). In Fig. 5B, organisms from the lung tissue of a rabbit treated with CAS1 had increased fragmentation of hyphal elements. As depicted in Fig. 5D and E, hyphae from rabbits treated with CAS6 had the greatest level of cell wall damage, as evidenced by increased fragmentation and vacuolization. Tissues from AMB-treated rabbits seldom revealed hyphal elements (Fig. 5F).
FIG. 5.
Effects of CAS and AMB in vivo on hyphal structures and microbiological clearance of Aspergillus fumigatus in experimental pulmonary aspergillosis. All specimens were stained with Grocott-Gomori’s methenamine-silver. Panels A to D demonstrate dose-dependent fragmentation, reduction in length, and increasing swelling and vacuolization of hyphal elements in a representative section of lung tissue from each dosage group. Panel E is a high magnification of the chlamydospore-like structure formed in CAS-treated hyphae. Panels: A, control; B, CAS1; C, CAS3; D and E, CAS6; F, AMB at a dose of 1 mg/kg/day. Magnification for panel E, ×800. Magnification for all other panels, ×320.
Antifungal prophylaxis.
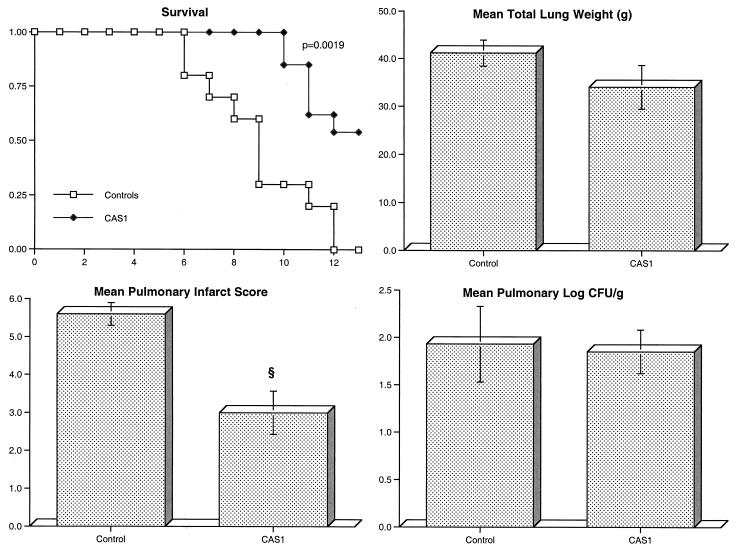
Rabbits receiving prophylactic CAS1 (n = 13) showed significant improvement in survival (P = 0.0019) and reduction in organism-mediated pulmonary injury as measured by infarct scores (P < 0.01), but again no effect on residual fungal burden was observed (Fig. 6).
FIG. 6.
Response of primary pulmonary aspergillosis in persistently neutropenic rabbits to prophylaxis as measured by survival, mean lung weight, mean pulmonary infarct score, and mean pulmonary tissue concentration of residual organisms (log CFU/gram) in untreated controls (n = 10) and rabbits treated with CAS1 (n = 13). Values are given as means ± SEMs (error bars). §, P < 0.01 (Mann-Whitney U test). For the measure of survival, the values on the x axis are days following inoculation and the values on the y axis are probability of survival.
Plasma pharmacokinetics of CAS.
The estimated plasma concentration-time profiles of CAS, the corresponding compartmental pharmacokinetic parameters, and the concentrations of CAS in lung tissue and BAL fluid are shown in Fig. 7 and Table 3. Dosages of 1, 3, and 6 mg/kg/day resulted in escalating peak levels in plasma that ranged from 21.85 ± 1.20 to 69.36 ± 6.23 μg/ml, greatly exceeding the MIC for the experimental isolate. At the 3- and 6-mg/kg/day dosage levels, mean levels in plasma remained above this MIC throughout the dosing interval. The concentrations of CAS in lung tissue increased in relation to increasing doses and exceeded the MIC. The levels of CAS in BAL fluid also increased in relation to dose and exceeded the MIC.
FIG. 7.
Concentration-time plots after multiple daily doses CAS1, CAS3, and CAS6 over 5 days. Each point plots the mean concentration ± SEM for 12 rabbits at each time point.
TABLE 3.
Estimated pharmacokinetic parameters of CAS and concentrations of CAS in plasma, lung tissue, and BAL fluida
| Parameterb | Result for treatment group:
|
P valuec | ||
|---|---|---|---|---|
| CAS1 | CAS3 | CAS6 | ||
| Cmax (μg/ml) | 21.85 ± 1.20 | 42.30 ± 2.75 | 69.36 ± 6.23 | <0.0001 |
| Cmin (μg/ml) | 0.089 ± 0.03 | 0.580 ± 0.13 | 1.34 ± 0.16 | <0.0001 |
| AUC0–24 (μg/ml · h) | 21.73 ± 2.73 | 77.65 ± 11.1 | 163.30 ± 14.78 | <0.0001 |
| AUC0–∞/dose (μg/ml · h) | 8.580 ± 1.45 | 12.01 ± 2.18 | 12.83 ± 1.31 | 0.1979 |
| Vss (liter/kg) | 0.325 ± 0.03 | 0.184 ± 0.01 | 0.094 ± 0.01 | <0.0001 |
| CL (liter/h/kg) | 0.052 ± 0.00 | 0.016 ± 0.00 | 0.007 ± 0.00 | <0.0001 |
| t1/2 (h) | 32.61 ± 0.72 | 37.34 ± 0.68 | 38.42 ± 0.86 | <0.0001 |
| Clung (μg/g) (range) | 1.300 ± 0.20 (0.41–2.55) | 5.82 ± 0.94 (1.11–11.9) | 7.68 ± 1.67 (1.45–23.9) | 0.0010 |
| CBAL (μg/ml) (range) | 0.014 ± 0.014 (0.00–0.10) | 0.302 ± 0.08 (0.00–0.86) | 0.563 ± 0.19 (0.00–1.41) | 0.0265 |
All values are expressed as means ± SEMs for 12 rabbits.
Cmin, concentration 24 h after dosing; CL, total clearance in plasma; t1/2, terminal elimination half-life; Clung, concentration of CAS in lung tissue; CBAL, concentration of CAS in BAL fluid.
P values were calculated by using ANOVA.
CAS was eliminated from plasma with a mean terminal half-life ranging from 32 to 38 h. CAS in these infected animals demonstrated a decrease in total clearance with increasing dosages (Table 3), corresponding to a dose-related decrease of the apparent volume of distribution at steady state (Vss). However, the dose-normalized AUC0–∞ did not demonstrate a significant difference across the dosage range.
Safety.
Table 4 demonstrates that rabbits treated with AMB had significant increases in their levels of creatinine and urea nitrogen in serum compared to those of CAS-treated rabbits or untreated controls (P < 0.001). There was no significant elevation of the levels of serum bilirubin or serum hepatic transaminases in any of the treatment groups
TABLE 4.
Effects of CAS and AMB on creatinine, urea nitrogen, ALT, AST, and bilirubin levels in the serum of persistently neutropenic rabbits with pulmonary aspergillosis
| Treatment group (n) | Level (mean ± SEM) in serum of:
|
||||
|---|---|---|---|---|---|
| Creatinine (mg/dl) | Urea nitrogen (mg/dl) | ALT (U/liter) | AST (U/liter) | Bilirubin (mg/dl) | |
| Control (12) | 0.95 ± 0.06 | 20.25 ± 1.41 | 89.08 ± 26.85 | 100.00 ± 60.45 | 0.27 ± 0.06 |
| CAS (35) | 0.98 ± 0.05 | 18.31 ± 1.27 | 66.43 ± 14.08 | 35.86 ± 9.40 | 0.32 ± 0.05 |
| AMB (6) | 2.00 ± 0.19a | 89.50 ± 12.60a | 58.67 ± 27.55 | 31.83 ± 25.44 | 0.12 ± 0.01 |
P < 0.001; calculated by using ANOVA with Bonferroni’s correction for multiple comparisons.
DISCUSSION
This study demonstrated that CAS administered therapeutically to persistently neutropenic rabbits with primary pulmonary aspergillosis improved survival and reduced organism-mediated pulmonary injury as measured by lung weight, pulmonary infarct score, and CT scan. These effects on survival and reduced pulmonary infarction were comparable to those of AMB. However, there was no improvement in organism clearance of A. fumigatus from the lung tissue (measured in log CFU/gram) in CAS-treated rabbits. By comparison, there was a significant reduction of residual fungal burden in AMB-treated animals. Despite the lack of an effect on the clearance of A. fumigatus from lung tissue, there was a dosage-dependent alteration in the cell wall morphology of Aspergillus hyphae in lung tissue. Corresponding to these microscopic findings, there was a concentration-dependent effect of CAS-induced hyphal damage measured by MTT assay. There was no evidence of hepatic or renal toxicity due to CAS. By comparison, AMB administered at a dose of 1 mg/kg/day caused significant renal impairment. When administered prophylactically, CAS significantly improved survival and reduced lung weight but again had no effect on residual fungal burden. CAS administered at doses from 1 to 6 mg/kg/day demonstrated linear plasma pharmacokinetics while maintaining concentrations in plasma above the MIC throughout the 24-h dosing interval at dosages of ≥3 mg/kg/day.
Pulmonary infarction and hemorrhagic necrosis due to angioinvasive hyphae are key elements in the pathogenesis of invasive pulmonary aspergillosis in profoundly neutropenic hosts (7). Organism-mediated pulmonary injury may be measured experimentally by several variables: total lung weight, pulmonary infarct lesions, and CT scan score. This study reveals that CAS interdicts the progression of organism-mediated pulmonary injury in comparison with that of the untreated controls. This effect appears to be comparable to that of AMB; however, the antifungal mechanisms are notably different.
As an echinocandin, CAS is a noncompetitive inhibitor of 1,3-β-d-glucan synthase activity (21, 22, 35, 36). Consistent with this mechanism of action was a concentration-dependent antifungal effect of hyphal damage in vitro and alteration of hyphal cell wall structure in vivo. However, individual damaged cellular units appear to be still viable as measured by the lack of reduction in fungal burden (measured in CFU/gram).
Douglas and colleagues have demonstrated that CAS preferentially destroys the apical cells and branching junctional cells of the hyphal structure of A. fumigatus (C. M. Douglas, J. C. Bowman, G. K. Abruzzo, A. M. Flattery, C. J. Gill, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, M. B. Kurtz, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1683, 2000). Other cellular units of the hyphal element may remain viable. Destruction of the junctional cells in the hyphal structure fragment the hyphal elements and lead to more CFU than the single hyphal element would have originally produced on quantitative cultures. The histological findings of hyphal fragmentation and the paradoxical rise in residual fungal burden (log CFU/gram) in our rabbit model are consistent with these in vitro findings of Douglas et al. and of Kurtz et al. (35; C. M. Douglas et al., 40th ICAAC). By comparison, AMB damages all cells in the hyphal structure.
These damaged cells treated with CAS appear histologically to have a reduced capacity to invade blood vessels. This reduction in angioinvasive potential coincides with reduced pulmonary infarct scores, lung weights, and CT scan scores, all of which are markers of organism-mediated pulmonary injury. The echinocandins are particularly novel in this regard, i.e., they may paradoxically increase the residual fungal burden while improving survival and reducing pulmonary injury due to A. fumigatus.
The deaths of rabbits in the AMB group are likely due to a combination of nephrotoxicity and invasive aspergillosis. The levels of creatinine in serum were higher in animals treated with AMB than in those treated with CAS. The rapid rates of hyphal injury and fungal cell death due to AMB are reflected in the lower fungal burden (measured in CFU/gram) as well as in the parameters indicating pulmonary injury (infarct scores and lung weights). CAS improved survival and did not cause nephrotoxicity. CAS reduced pulmonary injury (measured by reduced lung weights and pulmonary infarct scores), but it did so to a lesser extent than did AMB. This reduced rate of hyphal injury caused by CAS in vivo correlates with a reduced rate of hyphal damage in vitro in comparison to that caused by AMB, as shown in Fig. 1. A slower rate of hyphal injury by CAS may permit more time for angioinvasion and pulmonary infarction and, hence, reduced survival. Thus, there may be reduced survival due to nephrotoxicity in AMB-treated rabbits and reduced survival due to organism-mediated pulmonary infarction, possibly related to a slower rate of hyphal injury, in CAS-treated animals.
Host response may have a critical role in the effect of echinocandins against invasive aspergillosis. For example, Chiller et al. recently demonstrated that human phagocytic cells collaborate with CAS to enhance its antifungal activity against A. fumigatus (11). Brummer et al. found similar properties between micafungin and human phagocytes (8). The persistent and profound neutropenia in our rabbit model abrogates any contribution of neutrophils to the antifungal effect of CAS. Consequently, the microbiologic findings in this model are consistent with in vitro findings in which one also observes microcolonies and fragmented hyphal elements.
The levels of galactomannan in serum also paralleled the microbiologic increase in residual fungal burden of A. fumigatus in lung tissue (38, 48, 51). To our knowledge this is the first in vivo study to describe serial galactomannan levels in serum in invasive pulmonary aspergillosis in neutropenic hosts receiving an echinocandin. As serial galactomannan antigen levels may be used for therapeutic monitoring, one should be aware that profoundly neutropenic patients receiving echinocandins for aspergillosis might have persistent galactomannan antigenemia despite clinical improvement. Conversely, nonneutropenic hosts, who tend to clear A. fumigatus from their lungs when treated with an echinocandin, may be expected to have a corresponding decline in serum galactomannan antigenemia.
Optimal plasma sampling in infected animals on day 5 of antifungal treatment with CAS documented the maintenance of drug levels in plasma above the MIC for the experimental isolate throughout the dosing interval of 24 h in animals receiving the compound at dosages of ≥3 mg/kg/day. At dosages of 6 mg/kg/day, the mean AUC0–∞ and trough concentration of CAS exceeded the corresponding values measured after a single-dose administration of 70 mg to human volunteers while surpassing mean peak levels in plasma (J. A. Stone, C. H. Ballow, S. D. Holland, P. J. Deutsch, W. Hauck, E. Pequignot, M. Friel, T. Cicero, and J. B. Mccrea, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 853, 2000). This plasma exposure of CAS is at least similar to that achieved with dosages currently recommended for second-line treatment of invasive aspergillosis in patients. These data and the demonstration of substantial lung tissue concentrations make it appear unlikely that dosage regimen and/or differences in disposition were responsible for the lack of reduction of residual fungal burden by CAS (30).
In comparison to the previously reported compartmental plasma pharmacokinetics of CAS in healthy animals (26), neutropenic rabbits with lethal experimental invasive pulmonary aspergillosis exhibited a dose-dependent diminished clearance rate of CAS from plasma, associated with a higher AUC and a smaller Vss. Nevertheless, analysis of the dose-normalized AUC revealed dose-proportional exposure. These findings suggest that dose-related changes in AUC0–∞ and Vss reflected pseudononlinearity due to an inability to accurately measure below the lower limits of quantitation.
Differences in plasma pharmacokinetics between healthy and severely compromised animals also have been observed with anidulafungin (LY303366) (27) and may be explained by changes in blood volume, hematocrit, and/or protein binding as well as alterations in metabolism and excretion. Irrespective of their cause, these observations underscore the value of obtaining pharmacokinetic parameters from infected as well as healthy animals. Healthy animals permit intensive pharmacokinetic sampling and provide the a priori data for minimal sampling strategies in infected animals.
We conclude that CAS treatment in persistently neutropenic hosts improves and reduces organism-mediated pulmonary injury. This beneficial effect appears to be due to a dose-dependent effect on hyphal damage and fragmentation. Although these fragmented hyphae may paradoxically yield a higher fungal burden, the damaged and disrupted hyphae histologically caused less angioinvasion and, hence, reduced pulmonary injury and improved survival. The paradoxical rise of galactomannan antigen levels may be an important serological correlate of the increased residual fungal burden (measured in log CFU/gram).
Acknowledgments
We thank Joanne Peter for determining MICs and MFCs, the radiology technology staff of the NIH for performing CT scans, and Jeffrey Grove at Merck Sharp & Dhome-Chibret for expert assistance with the analytical assay.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):S43–S53. [DOI] [PubMed] [Google Scholar]
- 4.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi, F., A. M. Schimizzi, A. W. Fothergill, G. Scalise, and M. G. Rinaldi. 1999. In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 18:302–304. [DOI] [PubMed] [Google Scholar]
- 6.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenguer, J., M. C. Allende, J. W. Lee, K. Garrett, C. Lyman, N. M. Ali, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1995. Pathogenesis of invasive pulmonary aspergillosis during persistent granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Resp. Crit. Care Med. 152:1079–1086. [DOI] [PubMed] [Google Scholar]
- 8.Brummer, E., S. D. Chauhan, and D. A. Stevens. 1999. Collaboration of human phagocytes with LY 303366 for antifungal activity against Aspergillus fumigatus. J. Antimicrob. Chemother. 43:491–496. [DOI] [PubMed] [Google Scholar]
- 9.Caillot, D., J. F. Couaillier, A. Bernard, O. Casasnovas, D. W. Denning, L. Mannone, J. Lopez, G. Couillault, F. Piard, O. Vagner, and H. Guy. 2001. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 19:253–259. [DOI] [PubMed] [Google Scholar]
- 10.Caillot, D., O. Casasnovas, A. Bernard, J. F. Couaillier, C. Durand, B. Cuisenier, E. Solary, F. Piard, T. Petrella, A. Bonnin, G. Couillault, M. Dumas, and H. Guy. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J. Clin. Oncol. 15:139–147. [DOI] [PubMed] [Google Scholar]
- 11.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2001. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 39:99–103. [DOI] [PubMed] [Google Scholar]
- 12.De Bock, R. 1994. Epidemiology of invasive fungal infections in bone marrow transplantation. Bone Marrow Transplant. 14(Suppl. 5):S1–S2. [PubMed] [Google Scholar]
- 13.Debono, M., and R. S. Gordee. 1994. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 48:471–497. [DOI] [PubMed] [Google Scholar]
- 14.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608–615. [DOI] [PubMed] [Google Scholar]
- 16.Denning, D. W. 1997. Echinocandins and pneumocandins–a new antifungal class with a novel mode of action. J. Antimicrob. Chemother. 40:611–614. [DOI] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, Jr., A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Schelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356–368. [DOI] [PubMed] [Google Scholar]
- 20.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308–329. [DOI] [PubMed] [Google Scholar]
- 21.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs. 10:269–280. [DOI] [PubMed] [Google Scholar]
- 22.Georgopapadakou, N. H., and J. S. Tkacz. 1995. The fungal cell wall as a drug target. Trends Microbiol. 3:98–104. [DOI] [PubMed] [Google Scholar]
- 23.Graybill, J. R., L. K. Najvar, E. M. Montalbo, F. J. Barchiesi, M. F. Luther, and M. G. Rinaldi. 1998. Treatment of histoplasmosis with MK-0991 (L-743,872). Anitmicrob. Agents Chemother. 42:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graybill, J. R., L. K. Najvar, M. F. Luther, A. W. Fothergill. 1997. Treatment of murine disseminated candidiasis with L-743,872. Antimicrob. Agents Chemother. 41:1775–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graybill, J. R., R. Bocanegra, M. Luther, A. Fothergill, and M. J. Rinaldi. 1997. Treatment of murine Candida krusei or Candida glabrata infection with L-743,872. Antimicrob. Agents Chemother. 41:1937–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groll, A. H., B. M. Gullick, R. Petraitiene, V. Petraitis, M. Candelario, S. C. Piscitelli, and T. J. Walsh. 2001. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob. Agents Chemother. 45:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groll, A. H., D. Mickiene, R. Petraitiene, V. Petraitis, A. Field-Ridley, M. Candelario, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): a reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll, A. H., M. Kurtz, W. Schneider, V. Witt, H. Schmidt, M. Schneider, and D. Schwabe. 1999. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses 42:431–442. [DOI] [PubMed] [Google Scholar]
- 29.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343–500. [DOI] [PubMed] [Google Scholar]
- 30.Hajdu, R., R. Thompson, J. G. Sundelof, B. A. Pelak, F. A. Bouffard, J. F. Dropinski, and H. Kropp. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hruban, R. H., M. A. Meziane, E. A. Zerhouni, P. S. Wheeler, J. S. Dumler, and G. M. Hutchins. 1987. Radiologic-pathologic correlation of the CT halo sign in invasive pulmonary aspergillosis. J. Comput. Assisted Tomogr. 11:534–536. [DOI] [PubMed] [Google Scholar]
- 32.Krishnarao, T. V., and J. N. Galgiani. 1997. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhlman, J. E., E. K. Fishman, and S. S. Siegelman. 1985. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology 157:611–614. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz, M. B., and C. M. Douglas. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79–86. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitz, S. M., and R. D. Diamond. 1985. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 152:938–944. [DOI] [PubMed] [Google Scholar]
- 37.Lozano-Chiu, M., P. W. Nelson, V. L. Paetznick, and J. H. Rex. 1999. Disk diffusion method for determining susceptibilities of Candida spp. to MK-0991. J. Clin. Microbiol. 37:1625–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maertens, J., J. Verhaegen, H. Demuynck, P. Brock, G. Verhoef, P. Vandenberghe, J. Van Eldere, L. Verbist, and M. Boogaerts. 1999. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 37:3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 32:33–37. [DOI] [PubMed] [Google Scholar]
- 40.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 41.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 42.Nelson, P. W., M. Lozano-Chiu, and J. H. Rex. 1997. In vitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J. Med. Vet. Mycol. 35:285–287. [PubMed] [Google Scholar]
- 43.Onishi, J., M. Meinz, J. Thompson, J. Curotto, S. Dreikorn, M. Rosenbach, C. Douglas, G. Abruzzo, A. Flattery, L. Kong, A. Cabello, F. Vicente, F. Pelaez, M. T. Diez, I. Martin, G. Bills, R. Giacobbe, A. Dombrowski, R. Schwartz, S. Morris, G. Harris, A. Tsipouras, K. Wilson, and M. B. Kurtz. 2000. Discovery of novel antifungal (1,3)-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 44:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pannuti, C., R. Gingrich, M. A. Pfaller, C. Kao, and R. P. Wenzel. 1992. Nosocomial pneumonia in patients having bone marrow transplant: attributable mortality and risk factors. Cancer 69:2653–2662. [DOI] [PubMed] [Google Scholar]
- 45.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingfffard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Aspergillus Study Group. Medicine (Baltimore) 79:250–260. [DOI] [PubMed] [Google Scholar]
- 46.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus. Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251–255. [DOI] [PubMed] [Google Scholar]
- 47.Powles, M. A., P. Liberator, J. Anderson, Y. Karkhanis, J. F. Dropinski, F. A. Bouffard, J. M. Balkovec, H. Fujioka, M. Aikawa, D. McFadden, and D. Schmatz. 1998. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob. Agents Chemother. 42:1985–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stynen, D., A. Goris, J. Sarfati, and J. P. Latge. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verweij, P. E., D. Stynen, A. J. M. M. Rijs, B. E. de Pauw, J. A. A. Hoogkamp-Korstanje, and J. F. G. M. Meis. 1995. Sandwich enzyme-linked immunosorbent assay compared with pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J. Clin. Microbiol. 33:1912–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh, T. J., C. McEntee, and D. M. Dixon. 1987. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J. Clin. Microbiol. 25:931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh, T. J., J. W. Hiemenz, and E. Anaissie. 1996. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect. Dis. Clin. N. Am. 10:365–400. [DOI] [PubMed] [Google Scholar]
- 54.Walsh, T. J., K. Garrett, E. Feuerstein, M. Girton, M. Allende, J. Bacher, A. Francesconi, R. Schaufele, and P. A. Pizzo. 1995. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob. Agents Chemother. 39:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335–347. [PubMed] [Google Scholar]
- 56.Walsh, T. J., J. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 38:467–471. [PubMed] [Google Scholar]
- 57.Yamaoka, K. T., T. Nakagawa, and T. Uno. 1978. Application of Akaike’s information criterion in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165–175. [DOI] [PubMed] [Google Scholar]