Abstract
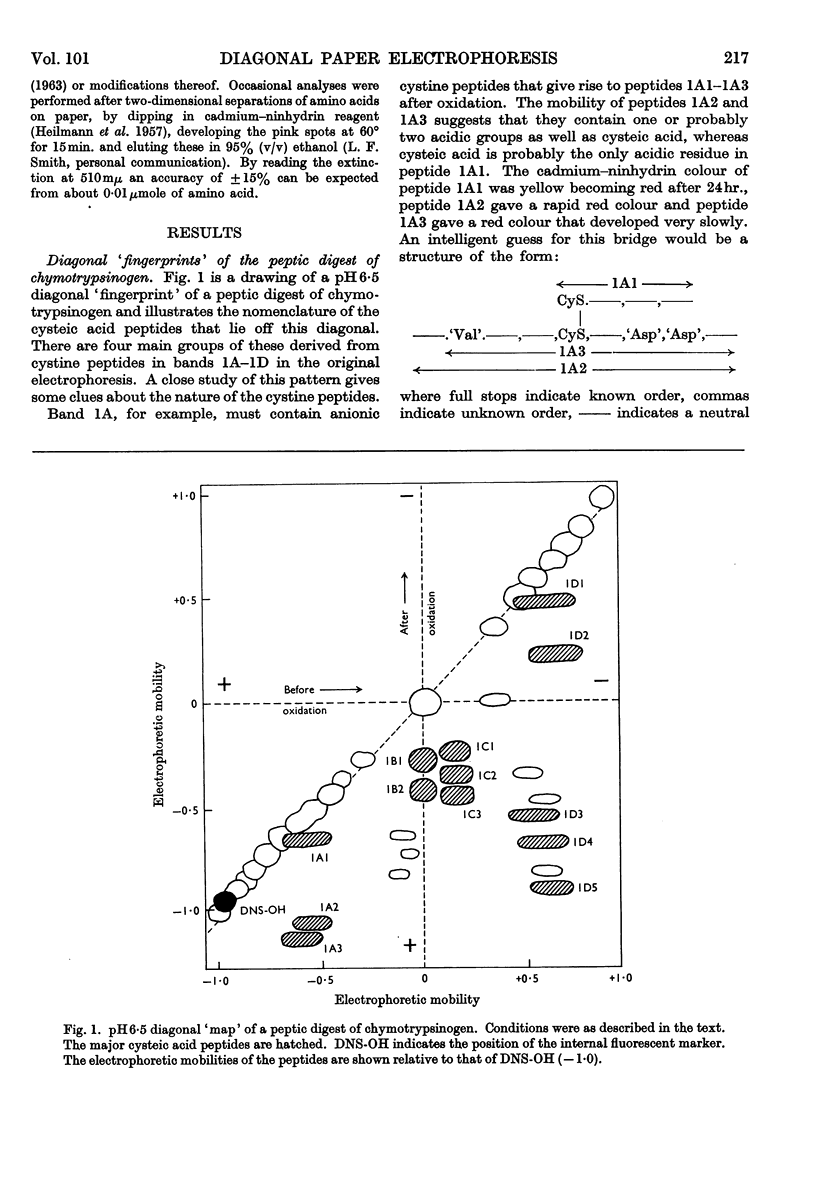
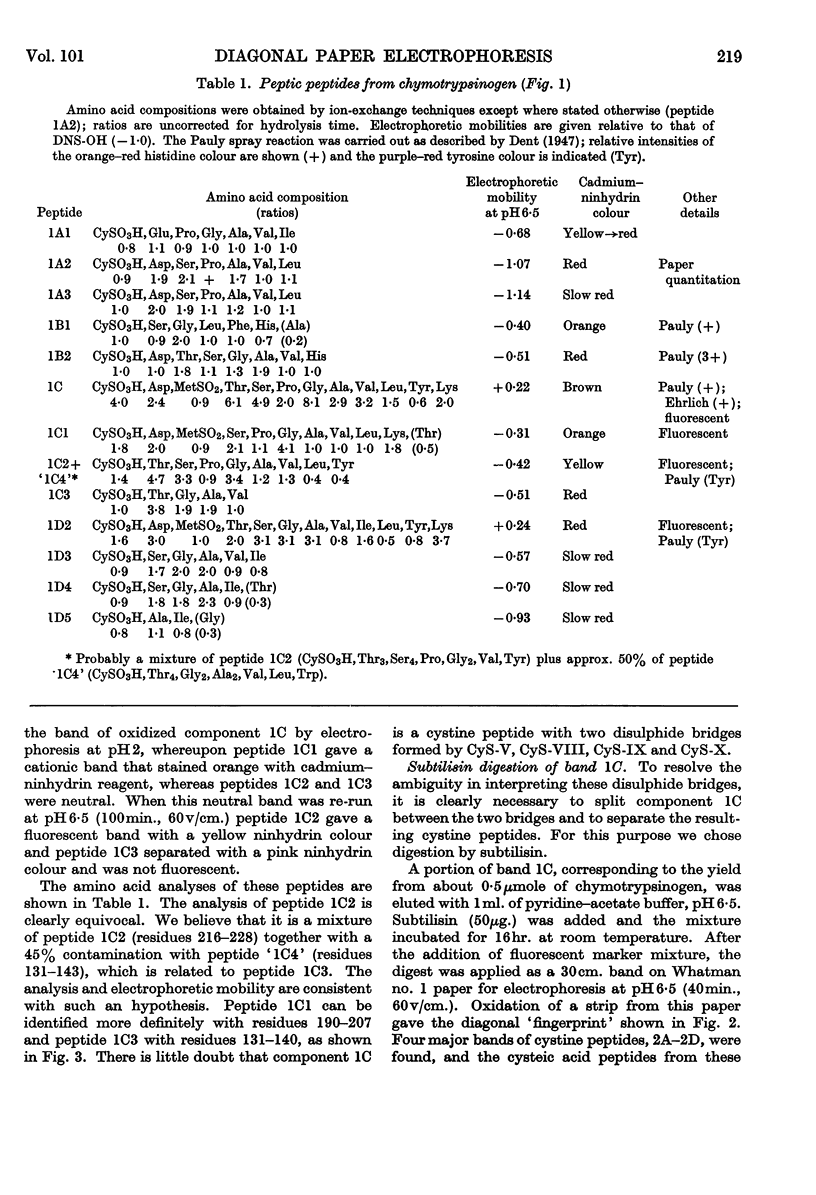
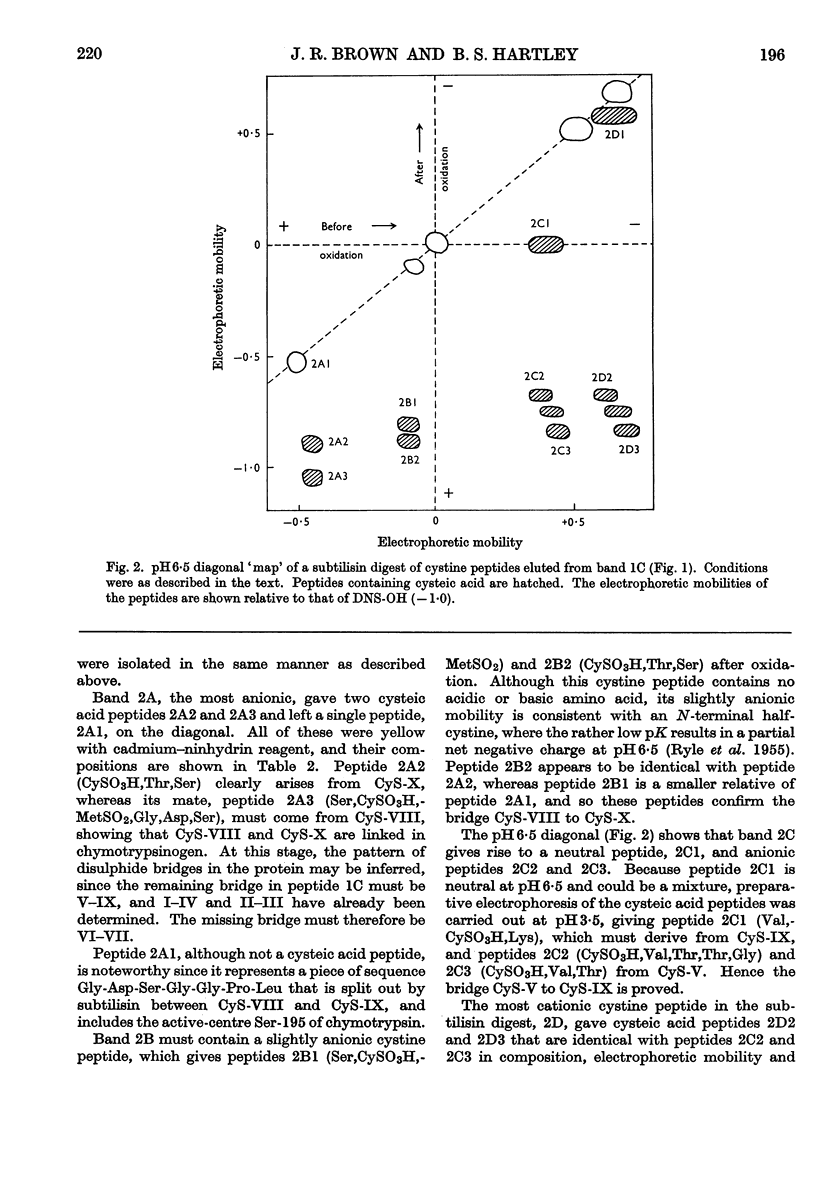
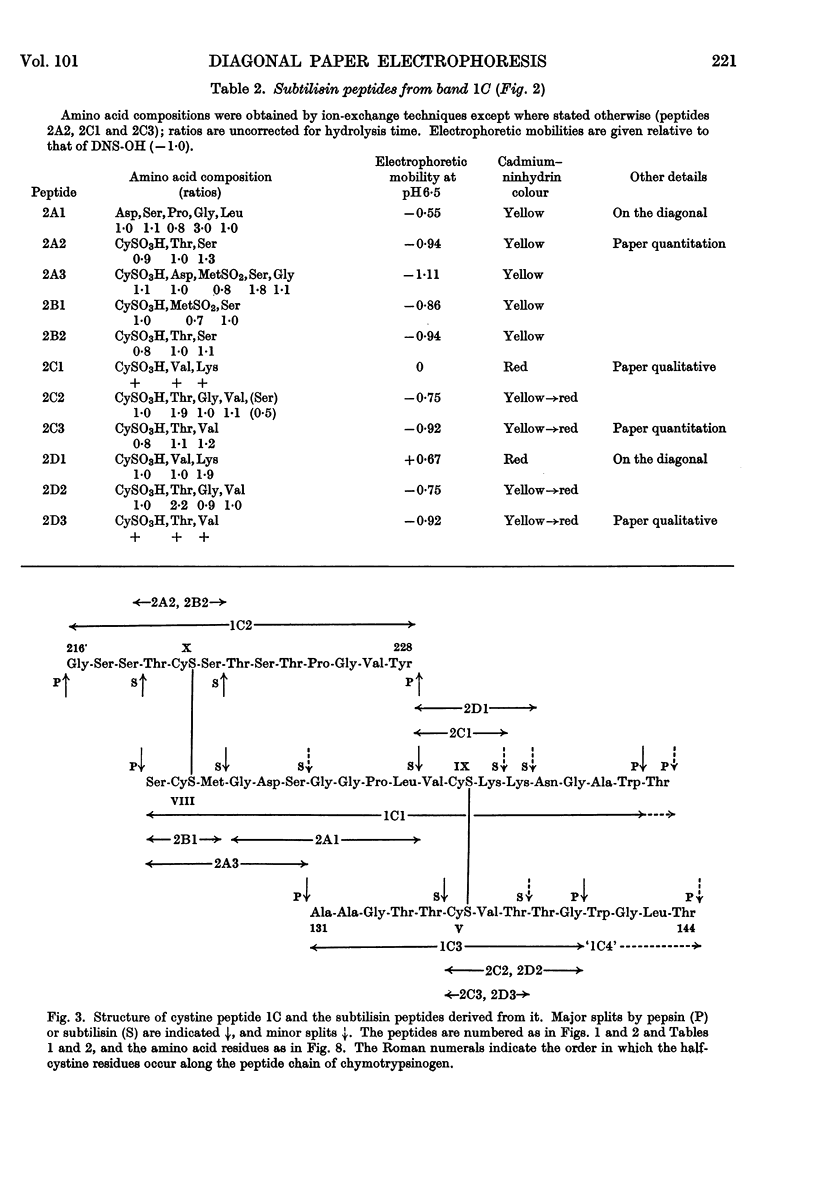
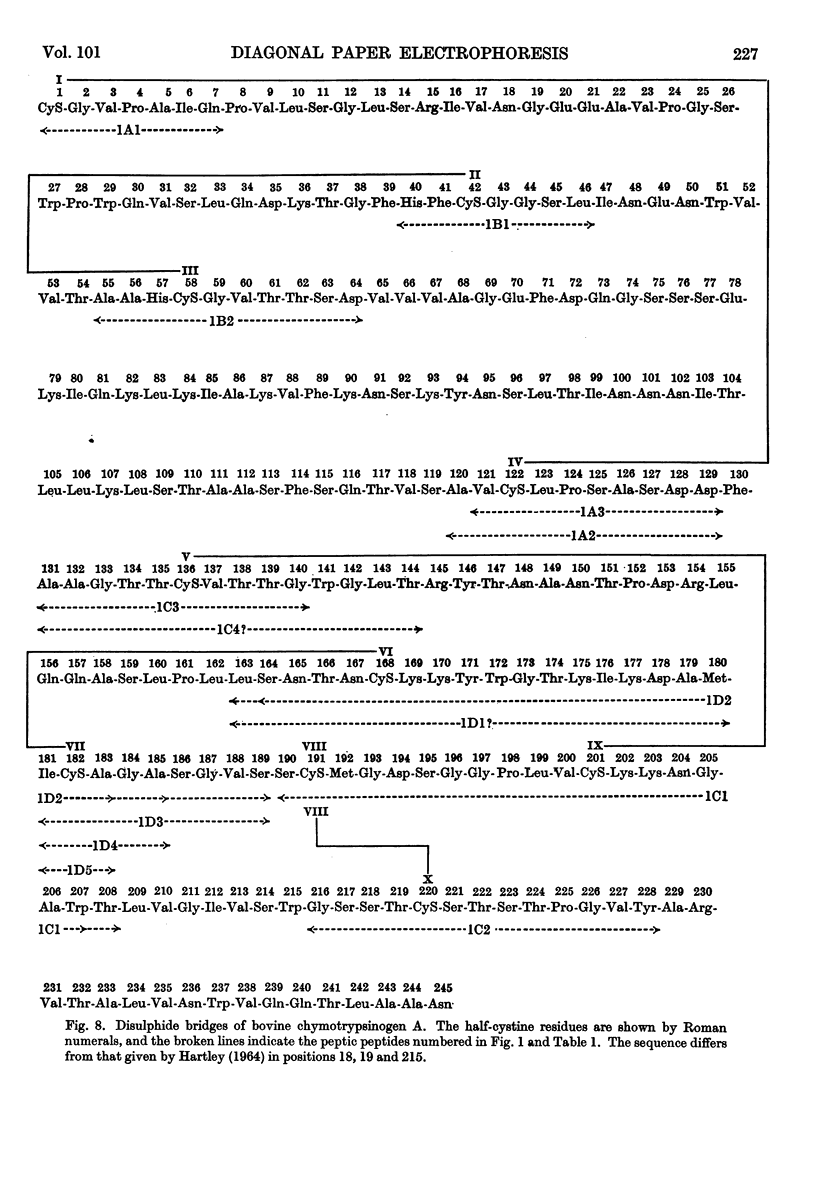
1. A new method is described for `fingerprinting' cysteic acid peptides derived from the disulphide bridges of proteins. Cystine peptides are separated by paper electrophoresis and oxidized on paper by performic acid vapour. Electrophoresis at right angles to the first direction produces parallel groups of cysteic acid peptides lying off a diagonal. This `fingerprint' reveals the way in which the cysteic acid peptides were originally joined in the protein. 2. The method allows a very easy selective purification of cysteic acid peptides. 3. By applying this method to bovine chymotrypsinogen A, we found that the half-cystine residues were linked 1–122, 42–58, 136–201, 168–182 and 191–220.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- HUNT J. A., OTTENSEN M. A comparison of three proteinases from various strains of Bacillus subtilis. Biochim Biophys Acta. 1961 Apr 1;48:411–412. doi: 10.1016/0006-3002(61)90498-x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- KEIL B., PRUSIK, SORM F. DISULPHIDE BRIDGES AND A SUGGESTED STRUCTURE OF CHYMOTRYPSINOGEN. Biochim Biophys Acta. 1963 Nov 15;78:559–561. doi: 10.1016/0006-3002(63)90927-2. [DOI] [PubMed] [Google Scholar]
- KEIL B. THE CHEMISTRY AND STRUCTURE OF PEPTIDES AND PROTEINS. Annu Rev Biochem. 1965;34:175–208. doi: 10.1146/annurev.bi.34.070165.001135. [DOI] [PubMed] [Google Scholar]
- KRAUT J. STRUCTURAL STUDIES WITH X-RAYS. Annu Rev Biochem. 1965;34:247–268. doi: 10.1146/annurev.bi.34.070165.001335. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L. The disulphide bridges of trypsin. J Mol Biol. 1965 Jul;12(3):929–932. doi: 10.1016/s0022-2836(65)80340-0. [DOI] [PubMed] [Google Scholar]
- MEEDOM B. The sequence of the thirteen amino acid residues in fraction A from oxidized alpha-chymotrypsin. Biochim Biophys Acta. 1958 Nov;30(2):429–430. doi: 10.1016/0006-3002(58)90072-6. [DOI] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., HAGOPIAN H. Some applications of two-dimensional ionophoresis. Anal Biochem. 1962 Apr;3:276–284. doi: 10.1016/0003-2697(62)90111-2. [DOI] [PubMed] [Google Scholar]
- NIEMANN C. ALPHA-CHYMOTRYPSIN AND THE NATURE OF ENZYME CATALYSIS. Science. 1964 Mar 20;143(3612):1287–1296. [PubMed] [Google Scholar]
- RYLE A. P., ANFINSEN C. B. Studies on the disulfide bridges in ribonuclease. Biochim Biophys Acta. 1957 Jun;24(3):633–635. doi: 10.1016/0006-3002(57)90258-5. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPACKMAN D. H., STEIN W. H., MOORE S. The disulfide bonds of ribonuclease. J Biol Chem. 1960 Mar;235:648–659. [PubMed] [Google Scholar]


