Abstract
FR160, a catechol iron chelator, and tetracyclines or norfloxacin exert in vitro additive or synergistic activity against a chloroquine-resistant Plasmodium falciparum clone. FR160 shows antagonistic effects in association with macrolides, ofloxacin, and rifampin.
The emergence and spread of parasite resistance to currently used antimalarial drugs indicates that novel compounds need to be discovered and developed by identification of novel chemotherapeutic targets (18). Iron chelation therapy was considered a suitable treatment for various infectious diseases, including malaria (10). Iron is needed for catalysis of DNA synthesis and for a variety of enzymes involved in electron transport and energy metabolism. Recent experimental observations obtained in vitro (9, 25), in rodent (6) and primate (19) models, and in clinical studies (8, 15) showed antimalarial activity of desferrioxamine. However, the time window of action of desferrioxamine is relatively limited, and the antimalarial activity is slow to develop, even after continuous in vitro or in vivo exposure to desferrioxamine (4). Various iron chelators were assessed to improve the drug lipophilicity, leading to increased access of drug to intracellular parasites and to faster action (13, 14). In addition, several studies explored the possibility of improving the antimalarial efficacy of iron chelators by using them in various combinations of iron chelators with different speeds of action, stage dependences, and degrees of reversibility of effects (7, 27, 28). The in vitro activity of FR160, a catecholate siderophore derived from spermidine, was shown previously (21). Use of combinations of antimalarials that do not have the same resistance mechanisms will reduce the chance of selection because the chance of a resistant mutant surviving is the product of the per-parasite mutation rates for the individual drugs multiplied by the number of parasites in an infection that are exposed to the drugs (29).
In this study, we assessed the combined action of FR160 with antibiotics against Plasmodium falciparum.
Parasites.
When required for drug assays, the chloroquine-resistant clone W2 (Indochina) was synchronized by sorbitol lysis (12). Susceptibilities to FR160 and antibiotics were determined after suspension in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% human serum (pooled from different A+ or AB sera from nonimmune donors) and buffered with 25 mM HEPES and 25 mM NaHCO3 (hematocrit of 1.5% and parasitemia of 0.5%).
Drugs.
The synthesis of FR160 was previously described (21). All antibiotics were obtained from Sigma Chemical (St. Louis, Mo.). Stock solutions were prepared in methanol for FR160, tetracycline, doxycycline, minocycline, oxytetracycline, erythromycin, spiramycin, roxithromycin, and rifampin and in dimethyl sulfoxide for ofloxacin, norfloxacin, and rolitetracycline. Twofold serial dilutions were prepared in sterile distilled water or RPMI for all these drugs. Final concentrations were distributed in triplicate into Falcon 96-well flat-bottom plates (Becton Dickinson, Franklin Lakes, N.J.).
In vitro assay.
The isotopic microdrug test used in this study was described previously (20). The 50% inhibitory concentration (IC50), i.e., the drug concentration corresponding to 50% of the uptake of [3H]hypoxanthine by the parasites in drug-free control wells, was determined by nonlinear regression analysis of log dose-response curves. Data were analyzed after logarithmic transformation and expressed as the geometric mean IC50s, and 95% confidence intervals (95% CI) were calculated.
Drugs combination.
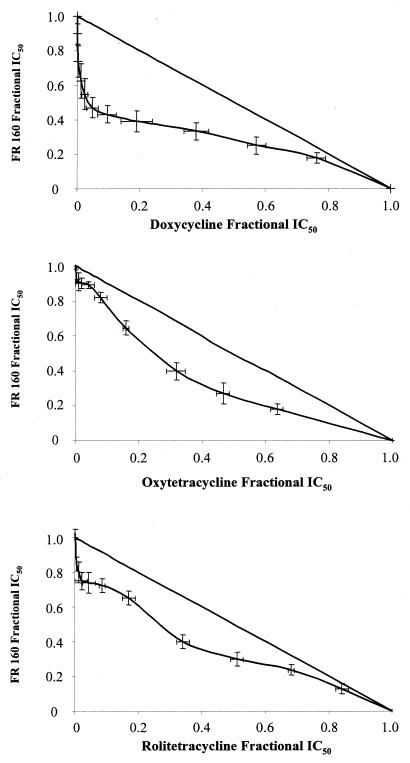
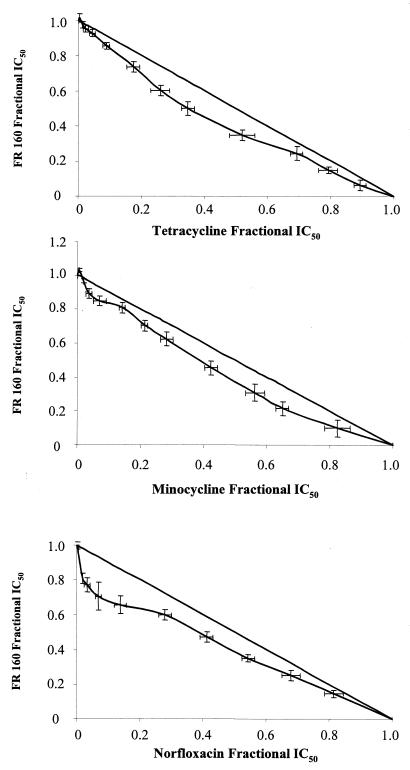
Combinations of FR160 with antibiotics were tested five independent times against the P. falciparum W2 clone. To evaluate drug interactions, isobolograms were constructed by plotting a pair of fractional IC50s for each combination of FR160 and antibiotics. The fractional IC50 for different antibiotics was calculated by dividing their fixed concentrations (12 to 16 concentrations) by the IC50 of tested drugs alone and plotted on the horizontal axis. The corresponding FR160 fractional IC50 was calculated by dividing the IC50 of FR160 combined with fixed concentrations of antibiotics and plotted on the vertical axis. A curve was then constructed through the resulting pairs of fractions from the ends of both the ends of both axes on the graph. Points above the straight diagonal line (corresponding to the points where there is no interaction between the drugs) are antagonistic; points below the straight diagonal line are considered synergistic (3).
Results.
The intrinsic activities of FR160 and antibiotics are summarized in Table 1. Doxycycline shows clear synergy with FR160 (Fig. 1). Oxytetracycline, rolitetracycline, and norfloxacin show mild synergy. Minocycline and tetracycline are categorized as additive. The other antibiotics show antagonism.
TABLE 1.
In vitro activities of the iron chelator FR160 and different tetracyclines, macrolides, quinolones, and rifampin against the chloroquine-resistant clone W2
| Antibiotic | Mean IC50 (μM) | 95% CIa |
|---|---|---|
| FR160 | 1.01 | 0.90–1.12 |
| Doxycycline | 7.7 | 7.1–8.4 |
| Tetracycline | 33.8 | 26.4–43.3 |
| Minocycline | 16.0 | 11.8–21.6 |
| Oxytetracycline | 46.0 | 44.2–47.9 |
| Rolitetracycline | 45.5 | 35.2–58.8 |
| Norfloxacin | 74.6 | 70.7–78.8 |
| Ofloxacin | 112.5 | 105.1–120.3 |
| Rifampin | 1.3 | 1.0–1.5 |
| Erythromycin | 39.4 | 27.9–55.5 |
| Spiramycin | 20.9b | 18.6–23.5 |
| Roxithromycin | 18.6 | 14.6–23.6 |
Values are geometric means of 5 to 10 independent assays.
Units per milliliter.
FIG. 1.
In vitro combinations of FR160 with doxycycline, tetracycline, oxytetracycline, minocycline, rolitetracycline, norfloxacin, roxithromycin, and ofloxacin against the P. falciparum W2 clone.
DISCUSSION
Our results indicate that combinations of FR160 and tetracyclines and norfloxacin have synergistic or additive effects against P. falciparum parasites. We previously showed that the in vitro activities of these antibiotics were inhibited by iron(III) like desferrioxamine or FR160 (21, 22). The antibiotics which show antagonistic effects with FR160 are not inhibited by iron(III). Norfloxacin shows synergy, while ofloxacin shows antagonism [norfloxacin is inhibited by iron(III), while ofloxacin activity is independent of iron]. FR160 enhances heme-catalyzed oxidation of lipid membranes (unpublished data) and may act, like iron chelators, against multiple iron-requiring proteins, such as the ribonucleotide reductase (RNR) or the dihydroorotate dehydrogenase (DHOD) (16). The formation and stability of the tyrosyl free radical of the R2 small subunit of the mammalian RNR, involved in de novo pyrimidine synthesis, depend on the presence of the iron center (1). Therefore, any compound that reduces the intracellular concentration of available iron below a certain level inhibits RNR activity by preventing the activation of the newly synthesized apo-R2 and the regeneration of the R2 iron center when apo-R2 is formed after losing iron (17). The antimalarial mechanism of action of tetracyclines is unknown. However, it seems that tetracycline exerts its action through an effect on parasite mitochondria and mitochondrial protein synthesis (11). The activity of P. falciparum DHOD, a particulate electron transport-linked enzyme involved in de novo pyrimidine synthesis and an iron-dependent enzyme, was depressed when the parasite was cultured with tetracycline (24). Doxycycline reduced the levels of nucleoside 5′-triphosphates and 2′-deoxynucleoside 5′-triphosphates (30).
Since the mechanisms underlying the antimalarial actions of FR160 and tetracyclines, one can speculate about mechanism for synergy. Other potential targets of tetracyclines (e.g., apicoplast, which is a target for different antibiotics [5, 26]) and iron chelators (e.g., iron-dependent enzymes [16]) could be involved in synergistic effects.
Resistance to antimalarial drugs reinforces the idea that novel antimalarial drugs should not be used for monotherapy. Because of the relatively slow antimalarial action of antibiotics and enhanced activity after prolonged contact, they would be best administered in conjunction with a rapidly acting regimen. The properties of FR160 and the synergistic or additive effects of combined FR160 and tetracyclines, and especially doxycycline, which shows in vitro and in vivo activities (2, 23), are attractive. The evaluation of their in vivo antimalarial effects is now required.
Acknowledgments
This work was supported by la Délégation Générale pour l’Armement (contrat d’objectif 9810060), la Direction de la Recherche et de la Technologie/Services Techniques des Recherches et des Développements Technologiques (contrat 97/2509A), le Groupe de Recherche en Parasitologie 1077, and la Direction Centrale du Service de Santé des Armées.
REFERENCES
- 1.Barlow, T., R. Eliasson, A. Platz, P. Reichard, and B. M. Sjoberg. 1983. Enzymatic modification of tyrosine to a stable free radical in ribonucleotide reductase. Proc. Natl. Acad. Sci. USA 80:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baudon, D., G. Martet, B. Pascal, J. Bernard, A. Keundjian, and R. Laroche. 1999. Efficacy of daily antimalarial chemoprophylaxis in tropical Africa using either doxycycline or chloroquine-proguanil; study conducted in 1996 in the French Army. Trans. R. Soc. Trop. Med. Hyg. 93:1–2. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122–130. [DOI] [PubMed] [Google Scholar]
- 4.Cabantchik, Z. I., H. Glickstein, J. Golenser, M. Loyevsky, and A. Tsafack. 1996. Iron chelators: mode of action as antimalarials. Acta Haematol. 95:70–77. [DOI] [PubMed] [Google Scholar]
- 5.Fichera, M. E., and D. S. Ross. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407–409. [DOI] [PubMed] [Google Scholar]
- 6.Fritsch, G., J. Treumer, D. T. Spira, and A. Jung. 1985. Suppression of mouse infections by desferrioxamine B. Exp. Med. 60:171–174. [DOI] [PubMed] [Google Scholar]
- 7.Golenser, J., A. Tsafack, Y. Amichai, J. Libman, A. Shanzer, and Z. I. Cabantchik. 1995. Antimalarial action of hydroxamate-based iron chelators and potentiation of desferrioxamine action by reversed siderophores. Antimicrob. Agents Chemother. 39:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordeuk, V. R., P. E. Thuma, G. M. Brittenham, C. McLaren, D. Parry, A. Backentose, G. Biemba, R. Msiska, L. Holmes, E. McKinley, L. Vargas, R. Gilkeson, and A. A. Poltera. 1992. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N. Engl. J. Med. 327:1473–1477. [DOI] [PubMed] [Google Scholar]
- 9.Hershko, C., and T. E. A. Peto. 1988. Deferoxamine inhibition of malaria is independent of host iron status. J. Exp. Med. 168:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hider, R. C., and Z. Liu. 1997. The treatment of malaria with iron chelators. J. Pharm. Pharmacol. 49:59–64. [Google Scholar]
- 11.Kiatfuengfoo, R., T. Suthiphongchai, P. Prapunwattana, and Y. Yuthavong. 1989. Mitochondria as the site of action of tetracycline on Plasmodium falciparum. Mol. Biochem. Parasitol. 34:109–116. [DOI] [PubMed] [Google Scholar]
- 12.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420. [PubMed] [Google Scholar]
- 13.Lytton, S. D., B. Mester, J. Libman, A. Shanzer, and Z. I. Cabantchik. 1994. Mode of action of iron(III) chelators as antimalarials. II. Evidence for differential on parasite iron-dependent nucleic acid synthesis. Blood 84:910–915. [PubMed] [Google Scholar]
- 14.Lytton, S. D., B. Mester, I. Dayan, H. Glickstein, J. Libman, A. Shanzer, and I. Catbanchik. 1993. Mode of action of iron(III) chelators as antimalarials. I. Membrane permeation properties and cytotoxic activity. Blood 81:214–221. [PubMed] [Google Scholar]
- 15.Mabeza, G. F., G. Biemba, and V. R. Gordeuk. 1996. Clinical studies of iron chelators in malaria. Acta Haematol. 95:78–86. [DOI] [PubMed] [Google Scholar]
- 16.Mabeza, G. F., M. Loyevsky, V. R. Gordeuk, and G. Weiss. 1999. Iron chelation therapy for malaria: a review. Pharmacol. Ther. 81:53–75. [DOI] [PubMed] [Google Scholar]
- 17.Nyholm, S., G. J. Mann, A. G. Johansson, R. J. Bergeron, A. Gräslund, and L. Thelander. 1993. Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J. Biol. Chem. 268:26200–26205. [PubMed] [Google Scholar]
- 18.Olliaro, P., and D. Wirth. 1997. New targets for antimalarial drug discovery. J. Pharm. Pharmacol. 49:29–33. [Google Scholar]
- 19.Pollack, S., R. N. Rosan, D. E. Davidson, and A. Escajadillo. 1987. Desferrioxamine suppresses Plasmodium falciparum in Aotus monkeys. Proc. Soc. Exp. Biol. Med. 184:162–164. [DOI] [PubMed] [Google Scholar]
- 20.Pradines, B., M. Mabika Mamfoumbi, D. Parzy, M. Owono Medang, C. Lebeau, J. R. Mourou Mbina, J. C. Doury, and M. Kombila. 1999. In vitro susceptibility of African isolates of Plasmodium falciparum from Gabon to pyronaridine. Am. J. Trop. Med. Hyg. 60:105–108. [DOI] [PubMed] [Google Scholar]
- 21.Pradines, B., F. Ramiandrasoa, L. K. Basco, L. Bricard, G. Kunesch, and J. Le Bras. 1996. In vitro activities of novel catecholate siderophore against Plasmodium falciparum. Antimicrob. Agents Chemother. 40:2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pradines, B., C. Rogier, T. Fusai, J. Mosnier, W. Darries, E. Baret, and D. Parzy. 2001. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrob. Agents Chemother. 45:1746–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradines, B., A. Spiegel, C. Rogier, A. Tall, J. Mosnier, T. Fusai, J. F. Trape, and D. Parzy. 2000. Antibiotics for prophylaxis of Plasmodium falciparum infections: in vitro activity of doxycycline against Senegalese isolates. Am. J. Trop. Med. Hyg. 62:82–85. [DOI] [PubMed] [Google Scholar]
- 24.Prapunwattana, P., W. J. O’Sullivan, and Y. Yuthavong. 1988. Depression of Plasmodium falciparum dihydroorotate dehydrogenase activity in in vitro culture by tetracycline. Mol. Biochem. Parasitol. 27:119–124. [DOI] [PubMed] [Google Scholar]
- 25.Raventos-Suarez, C., S. Pollack, and R. L. Nagel. 1982. Plasmodium falciparum: inhibition of in vitro growth by desferrioxamine. Am. J. Trop. Med. Hyg. 31:919–922. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan, M., J. Li, S. Kumar, M. J. Rogers, and T. F. McCutchan. 2000. Effects of interruption of apicoplast on malaria infection, development, and transmission. Mol. Biochem. Parasitol. 109:17–23. [DOI] [PubMed] [Google Scholar]
- 27.Tsafack, A., J. Golenser, J. Libman, A. Shanzer, and Z. I. Cabantchik. 1995. Mode of action of iron(III) chelators as antimalarials. III. Overadditive effects in the combined action of hydroxamate-based agents on in vitro growth of Plasmodium falciparum. Mol. Pharmacol. 47:403–409. [PubMed] [Google Scholar]
- 28.Tsafack, A., M. Loyevsky, P. Ponka, and Z. I. Cabantchik. 1996. Mode of action of iron(III) chelators as antimalarials. IV. Potentiation of desferal action by benzoyl and isonicotinoyl hydrazone derivatives. J. Lab. Clin. Med. 127:574–582. [DOI] [PubMed] [Google Scholar]
- 29.White, N. 1999. Antimalarial drug resistance and combination chemotherapy. Phil. Trans. R. Soc. Lond. B 354:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo, A. E. T., K. H. Rieckmann, and R. I. Christopherson. 1998. Indirect inhibition by antibiotics of nucleotide and deoxynucleotide biosynthesis in Plasmodium falciparum. Southeast Asian J. Trop. Med. Public Health 29:24–26. [PubMed] [Google Scholar]