Abstract
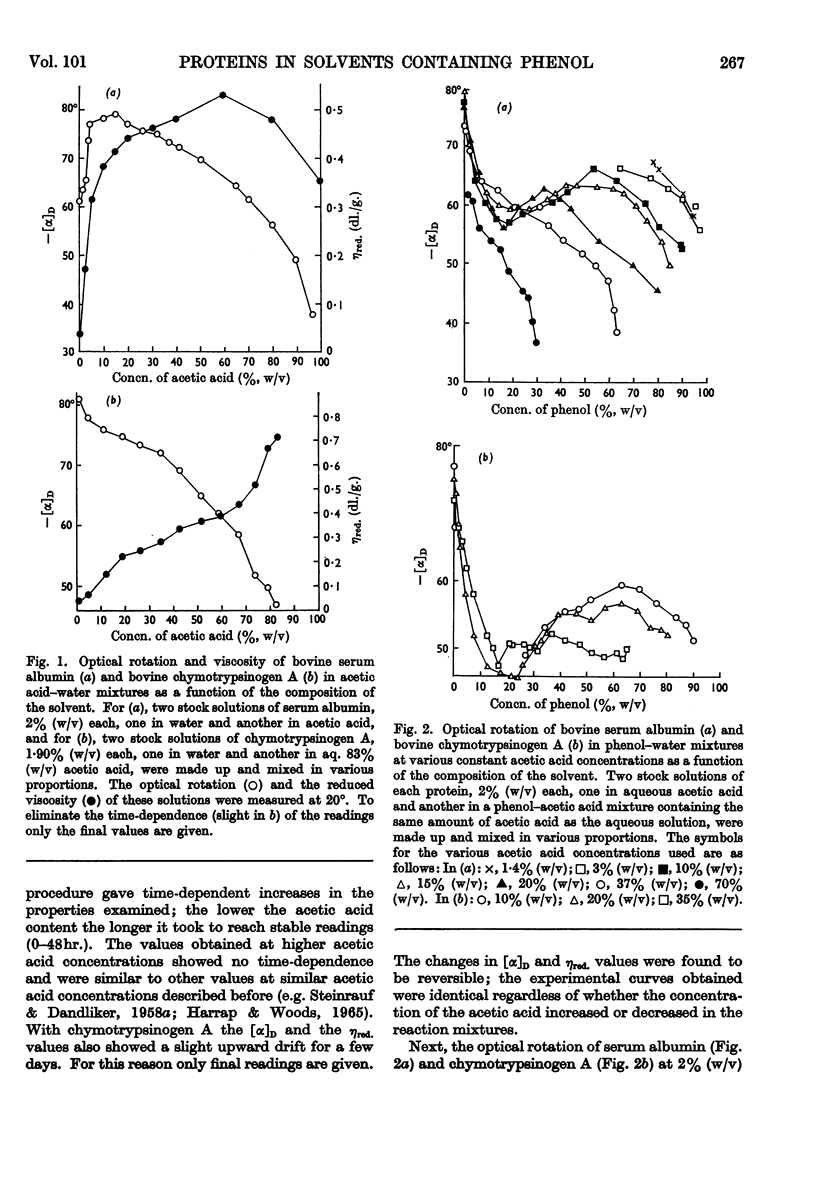
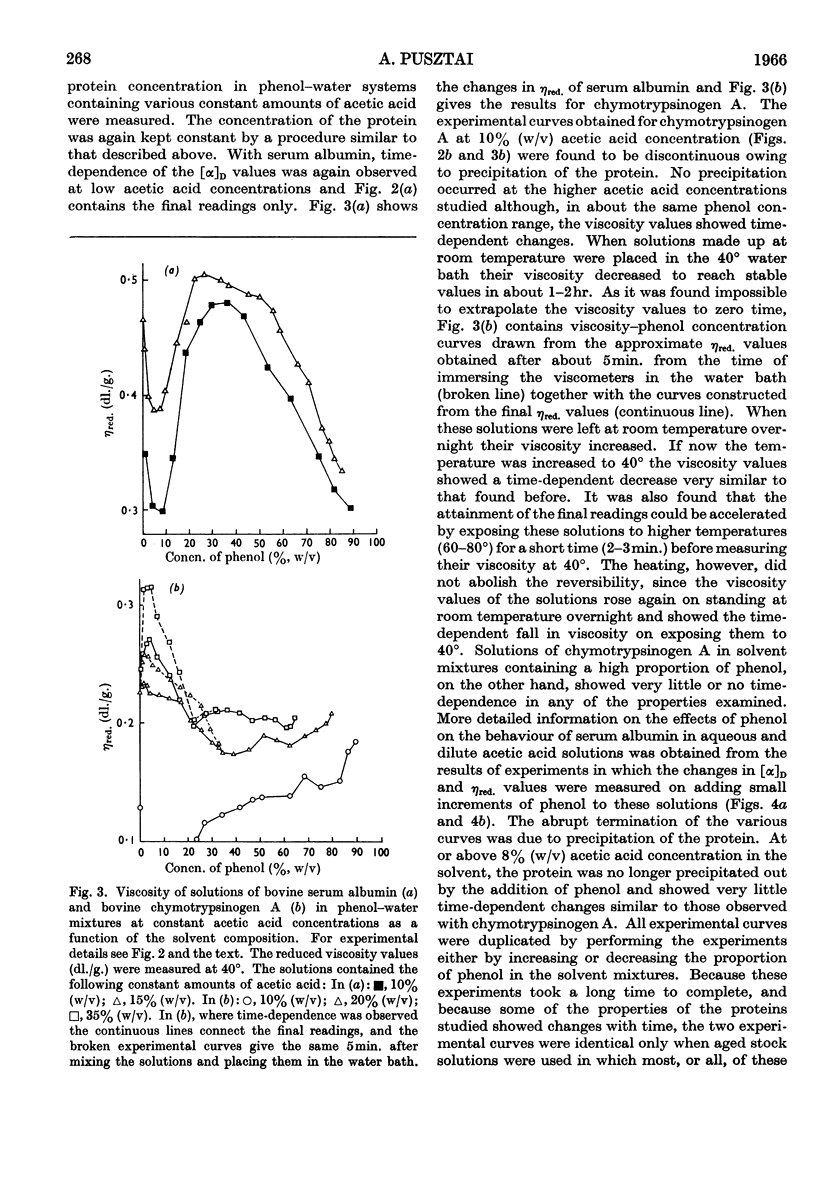
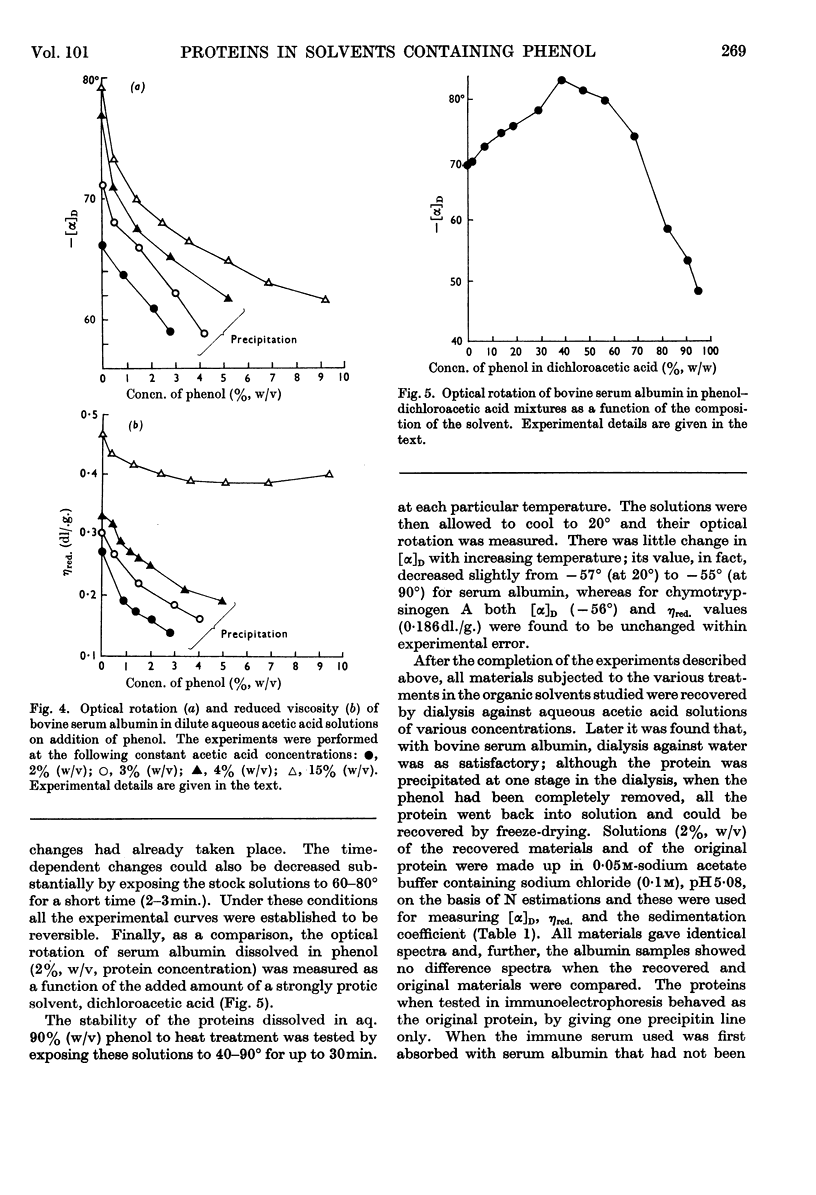
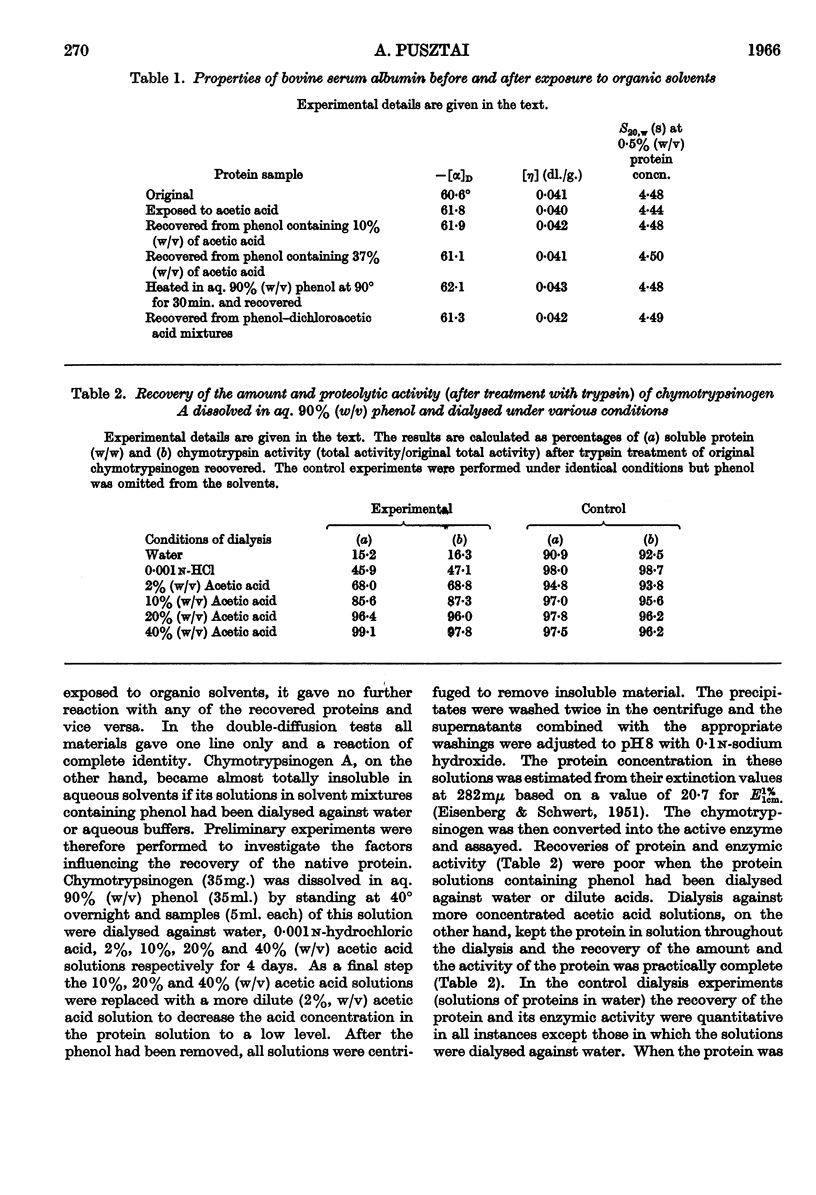
1. The optical rotation and reduced viscosity of bovine serum albumin and chymotrypsinogen A in solvents containing phenol, acetic acid and water were studied. 2. The changes brought about in the properties of the proteins by varying the composition of the solvent or by heat treatment in these solvents were established to be reversible. 3. A method for returning the proteins to aqueous media, based on these observations, was worked out. 4. The recovered proteins were shown to be very similar to, if not identical with, the native proteins on the basis of measurements of optical rotation, viscosity, sedimentation, ultraviolet spectroscopy, immunochemical behaviour (serum albumin) and proteolytic activity (chymotrypsinogen A, after activation with trypsin). 5. The importance of the findings for partitioning of polyelectrolytes in the phenol–aqueous buffer systems is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGELOW C. C., KRENITSKY T. A. THE INFLUENCE OF IONIC STRENGTH AND ORGANIC SOLVENTS ON ACID TRANSITIONS OF PROTEINS. Biochim Biophys Acta. 1964 Jul 29;88:130–141. doi: 10.1016/0926-6577(64)90161-5. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., Jr Reactivation of diethyl p-nitrophenyl phosphate-inhibited alpha-chymotrypsin by hydroxylamine. J Biol Chem. 1954 Mar;207(1):443–458. [PubMed] [Google Scholar]
- Cooper E. A. On the Relations of Phenol and Meta-Cresol to Proteins; A Contribution to our knowledge of the Mechanism of Disinfection. Biochem J. 1912;6(4):362–387. doi: 10.1042/bj0060362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E. A., Woodhouse D. L. On the Relations of the Phenols and their Derivatives to Proteins. A Contribution to our Knowledge of the Mechanism of Disinfection: Part IV. The Halogen Phenols. Biochem J. 1923;17(4-5):600–612. doi: 10.1042/bj0170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG M. A., SCHWERT G. W. The reversible heat denaturation of chymotrypsinogen. J Gen Physiol. 1951 May;34(5):583–606. doi: 10.1085/jgp.34.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLITZ D. G., DEKKER C. A. STUDIES ON A RIBONUCLEASE FROM USTILAGO SPHAEROGENA. I. PURIFICATION AND PROPERTIES OF THE ENZYME. Biochemistry. 1964 Oct;3:1391–1399. doi: 10.1021/bi00898a001. [DOI] [PubMed] [Google Scholar]
- HUPPERT J., PELMONT J. Ultracentrifuge studies of RNA degradation. Arch Biochem Biophys. 1962 Aug;98:214–223. doi: 10.1016/0003-9861(62)90175-3. [DOI] [PubMed] [Google Scholar]
- Harrap B. S., Woods E. F. Effect of different solvent systems on the aggregation and conformation of S-carboxymethyl bovine serum albumin. Biopolymers. 1965 Dec;3(6):595–607. doi: 10.1002/bip.360030601. [DOI] [PubMed] [Google Scholar]
- PUSZTAI A. STUDIES ON THE EXTRACTION OF NITROGENOUS AND PHOSPHORUS-CONTAINING MATERIALS FROM THE SEEDS OF KIDNEY BEANS (PHASEOLUS VULGARIS). Biochem J. 1965 Mar;94:611–616. doi: 10.1042/bj0940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. Interactions of proteins with other polyelectrolytes in a two-phase system containing phenol and aqueous buffers at various pH values. Biochem J. 1966 Apr;99(1):93–101. doi: 10.1042/bj0990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. R., JENCKS W. P. THE EFFECT OF COMPOUNDS OF THE UREA-GUANIDINIUM CLASS ON THE ACTIVITY COEFFICIENT OF ACETYLTETRAGLYCINE ETHYL ESTER AND RELATED COMPOUNDS. J Am Chem Soc. 1965 Jun 5;87:2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- RUSHIZKY G. W., GRECO A. E., HARTLEY R. W., Jr, SOBER H. A. Concentration and desalting of ribonucleases. Biochem Biophys Res Commun. 1963 Feb 18;10:311–314. doi: 10.1016/0006-291x(63)90530-8. [DOI] [PubMed] [Google Scholar]
- TANFORD C., DE P. K. The unfolding of beta-lactoglobulin at pH 3 by urea, formamide, and other organic substances. J Biol Chem. 1961 Jun;236:1711–1715. [PubMed] [Google Scholar]


