Abstract
The purpose of the present study was to characterize the partitioning of artemisinin into both uninfected and Plasmodium falciparum-infected red blood cells (RBCs). The partitioning of [14C](+)-artemisinin into RBCs was studied at four different hematocrit levels and eight time periods. At the optimum time of 2 h, the partitioning process was investigated with eight different drug concentrations ranging from 0.88 to 3.52 μM at 37 and 4°C. The effect of the presence of unlabeled artemisinin on the partitioning of the same concentration of [14C]artemisinin was studied. About 35 to 40% of the drug was seen to partition into uninfected RBCs at a hematocrit of 33%, irrespective of the incubation period or the drug concentration used. In contrast, infected RBCs showed an increase in partitioning of the drug with time until saturation was achieved at 1 h. While the partitioning of artemisinin into parasitized RBCs at 37°C was found to be significantly higher than that in nonparasitized RBCs, at 4°C both parasitized and nonparasitized RBCs showed identical partitioning of the drug. The partitioning of [14C]artemisinin into parasitized RBCs was completely inhibited in the presence of the same concentration of unlabeled artemisinin. However, no such effect was observed in nonparasitized cells, and no evidence suggesting that binding of the drug in parasitized RBCs is reversible was found. The partitioning of artemisinin into parasitized RBCs was found to be rapid, saturable, temperature dependent, irreversible, and subject to competitive inhibition with unlabeled artemisinin. The results obtained suggest the involvement of carrier mediation in the partitioning of artemisinin across the parasitized RBC membrane. In contrast, simple passive diffusion of artemisinin was seen in nonparasitized RBCs.
Artemisinin, a naturally occurring endoperoxide, has been shown to possess good antimalarial activity against drug-resistant strains of Plasmodium falciparum, the parasite responsible for most fatal malarial infections (14, 20, 22). The last two decades have seen the emergence of numerous structural analogs of artemisinin with better bioavailabilities, improved formulation properties, and enhanced antimalarial activities (6, 20, 26). The parasiticidal activity of the artemisinin class of compounds is thought to be due in part to the presence of the C—O—O—C endoperoxide bridge (3, 15, 20). The mode of action and biologic activity of the artemisinin class have been intensely investigated by various researchers, with the drug having been found to be selectively toxic to the malarial parasites (18, 19). One reason for this selective toxicity may be attributed to the increased accumulation of artemisinin-type drugs in parasitized red blood cells (RBCs) (1, 11, 21).
Most of these studies, however, have been carried out with the artemisinin analog dihydroartemisinin (1, 11). While dihydroartemisinin is a close structural analog of artemisinin, it nevertheless, unlike artemisinin, undergoes conversion to an aldehyde in aqueous media. The aldehyde moiety in turn can bind to proteins, forming a Schiff base-like compound, which may account for the increased accumulation of dihydroartemisinin seen in parasitized RBCs (1, 11). Even though an increased accumulation of artemisinin in parasitized cells has also been reported (20, 21), a need still exists to carry out a comprehensive study of the factors that control the partitioning of the parent compound artemisinin into parasitized RBCs.
The relevance of such an extensive characterization of the process of partitioning of artemisinin to the drug’s biologic activity and mode of action is immense. An overwhelming relevance of partitioning and accumulation studies to biologic activity, antimalarial potency, and mode of action has been shown for the 4-aminoquinolines chloroquine and amodiaquine (7, 12, 13, 16) as well as for other classes of antimalarials (17, 24). Not only is this study intended to provide further evidence pointing to the absence of a nonspecific passive partitioning of artemisinin across the parasitized RBC membrane, it is also intended to serve as a prelude to facilitate similar studies with select synthetic artemisinin analogs.
MATERIALS AND METHODS
Materials. (i) [14C]artemisinin.
[14C](+)-artemisinin (specific activity, 11.6 mCi/mmol) was synthesized by Avery et al. (2) at the Department of Medicinal Chemistry, University of Mississippi, with the label at the C-9 methyl (a metabolically stable position) on the lactone ring.
(ii) Uninfected and infected RBCs.
The D6 strain of P. falciparum was cultivated in human type A-positive RBCs obtained from Mississippi Blood Services, Jackson, and was subcultured daily by a modification of the method of Trager and Jenson (25). Culture suspensions of RBCs from the same unit as the uninfected RBCs were prepared in 10% malaria complete RPMI 1640 medium with 10 ml of heat-inactivated normal type A-positive human plasma in T-25 Corning tissue culture flasks. The flasks were gassed with a mixture that comprised 90% nitrogen, 5% oxygen, and 5% carbon dioxide and were incubated at 37°C for 42 to 44 h (to the late-ring or trophozoite stage). Prior to every experiment, the parasitemia was determined by using a thin blood film stained with Diff Quick Solutions I and II and was counted by using an Olympus BH-2 light microscope. The parasitemia was found to range between 6 and 7% in all studies.
(iii) Chemicals.
Toluene and isopropanol were obtained from Fisher Scientific, Fairlawn, N.J.; Dulbecco’s phosphate-buffered saline was from Sigma Chemical Company, St. Louis, Mo.; and hydrogen peroxide was purchased from Spectrum Quality Products Inc., Gardena, Calif., while Soluene-350 and Hionic Fluor were obtained from Packard, Meriden, Conn.
Methods. (i) Hematocrit selection.
Experiments were performed with 10, 20, 33, and 44% hematocrits with both uninfected and infected RBCs. The cells were incubated in triplicate for 2 h at 37°C with 0.88 μM artemisinin. The experimental protocol used was a modification of the one used by Gu et al. (11) for studies with [3H]dihydroartemisinin.
(ii) Incubation time effect.
Fresh uninfected RBCs were washed twice with Dulbecco’s phosphate-buffered saline and centrifuged at 3,000 rpm for 5 min in a Mistral 3000I centrifuge. The white blood cells and platelets were removed with a glass transfer pipette and discarded. Parasitized RBCs were centrifuged, and the culture medium was also discarded. The toluene from 14.3 μl of artemisinin was evaporated off under dry nitrogen and the drug was redissolved in 2.5 ml of RPMI 1640 medium. Then, 50 μl of the drug solution was further diluted with 420 μl of RPMI 1640 medium in a 1.5-ml Eppendorf microcentrifuge tube along with 230 μl of uninfected or infected RBCs. The final artemisinin concentration was 1.41 μM, with an RBC hematocrit of 33%. Duplicate samples were prepared and incubated in a Precision water bath at 37°C for eight different time periods that varied from 5 min to 3 h. At the end of the incubation period, the tubes were centrifuged at 13,000 × g for 3.5 min in a Fisher model 235C microcentrifuge to form an RBC pellet. The supernatant was removed and transferred to a 20-ml glass scintillation vial for analysis. The RBC pellets were lysed with 6 ml of cold 20 mosM hypotonic saline. A 400-μl aliquot was then transferred to a scintillation vial for analysis.
(iii) Drug concentration effect.
The effects of eight different drug concentrations ranging from 0.88 to 3.52 μM on the partitioning of artemisinin into parasitized and nonparasitized RBCs were investigated. Drug concentrations were chosen by using pharmacokinetic data detailing plasma drug levels (5, 8). This limited fourfold concentration range was chosen with a view to working strictly with therapeutic drug levels to try and mimic in vivo drug concentrations and pharmacokinetics. The samples were prepared with a 33% hematocrit in triplicate and were incubated for 2 h at 37°C in a shaker water bath. The samples were then processed as discussed above.
(iv) Temperature effect.
In order to assess whether active or passive partitioning processes are taking place, studies were performed at 4°C. Partitioning studies were carried out with four different drug concentrations ranging from 0.88 to 3.52 μM. The samples were incubated at 4°C for 2 h, and the partitioning data obtained were compared to the corresponding results obtained at 37°C.
(v) Competitive binding effect.
Triplicate samples of both nonparasitized and parasitized RBCs at 33% hematocrit were prepared as described above and incubated with 0.88 μM each [14C]artemisinin as well as unlabeled artemisinin at 37°C. The unlabeled artemisinin was added at three different stages: along with, 1 h prior to, and 1 h after the addition of [14C]artemisinin. The samples were incubated for a total of 1 h in the first case and 2 h in the last two cases. The partitioning of [14C]artemisinin into the RBCs in the presence of unlabeled artemisinin was calculated and compared to that for a control set of RBCs incubated under identical conditions with only 0.88 μM [14C]artemisinin.
(vi) Analysis of samples.
A 1.5-ml aliquot of a 50:50 mixture of isopropanol and Soluene-350 was added to each scintillation vial. The vials were incubated at 50°C for 30 min in a water bath. The samples were then bleached with 1.5 ml of 9% hydrogen peroxide and were again incubated at 50°C for 30 min. Finally, 15 ml of Hionic Fluor scintillation cocktail was added to each vial. The contents of the vials were mixed, and the counts in terms of the disintegrations per minute were obtained with a Packard liquid scintillation counter.
Assuming a linear correlation between the disintegrations per minute and the concentration of drug, a one-point calibration was performed. In a scintillation vial, 50 μl of drug solution was treated identically to the unknown samples. The disintegrations per minute obtained for the supernatant and the RBC pellets were then calculated as a percentage of the total disintegrations per minute, and a quantity was determined. Mass balance studies were also performed.
RESULTS
Hematocrit selection.
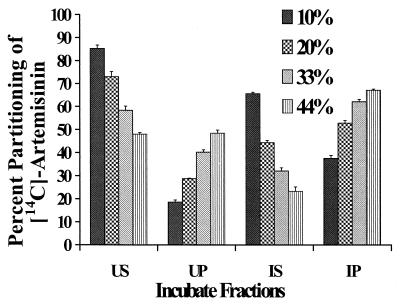
The partitioning of [14C]artemisinin into both nonparasitized and parasitized RBCs was seen to increase steadily with the hematocrit (Fig. 1). Parasitized RBCs showed an increased partitioning of the drug in comparison to that for nonparasitized RBCs. For example, with a hematocrit of 33%, only about 40% of the artemisinin partitioned into uninfected RBCs, while infected cells exhibited a drug partitioning of 62%. All mean partitioning values were found to be statistically different.
FIG. 1.
Results of studies with different hematocrits and [14C]artemisinin-uninfected supernatant (US), uninfected pellet (UP), infected supernatant (IS), and infected pellet (IP) with 6% parasitemia. Triplicate samples were incubated for 2 h at 37°C with a drug concentration of 0.88 μM. The error bars indicate the relative standard deviation [relative standard deviation = (standard deviation/mean) × 100].
Time course of partitioning.
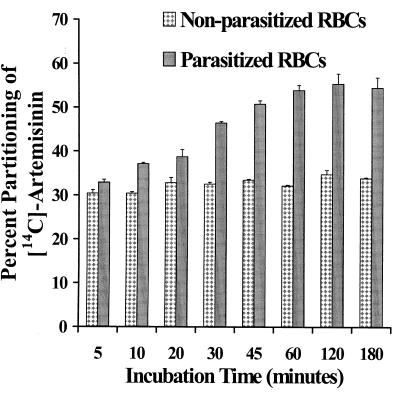
Uninfected RBCs showed a rapid partitioning (discernible partitioning of the drug even for the shortest incubation time period chosen) of the drug with respect to the amount of drug in the supernatant. Between 30 and 33% of the drug partitioned into nonparasitized cells at all time periods (Fig. 2). In contrast, the partitioning of the drug into parasitized RBCs increased steadily with time up to 45 min, when a level of over 50% partitioning of the drug was achieved, and with increasing time no statistically different results were noted (Fig. 2). Mass balance studies showed that 90 to 100% of the added drug was accounted for.
FIG. 2.
Partitioning of [14C]artemisinin in nonparasitized and parasitized (7% parasitemia) RBCs at eight different incubation time periods ranging from 5 min to 3 h. Duplicate samples with 33% hematocrit were prepared and incubated at 37°C with a drug concentration of 1.41 μM. The error bars indicate the relative standard deviation [relative standard deviation = (standard deviation/mean) × 100].
Effect of artemisinin concentration on partitioning.
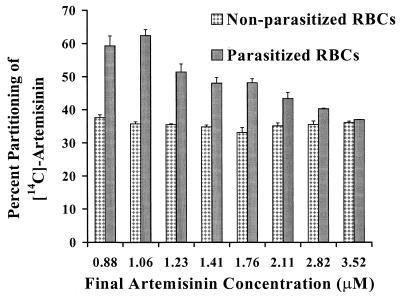
Uninfected RBCs incubated with eight different drug concentrations ranging from 0.88 to 3.52 μM for 2 h at 37°C partitioned a constant 37 to 40% of the drug (Fig. 3). In contrast, infected RBCs showed almost 60% partitioning of artemisinin when drug concentrations of 0.88 and 1.06 μM were used, and the percentage of the drug partitioned between the supernatant and RBCs as a function of the total amount of drug present was found to decline steadily with increasing concentration (Fig. 3). All mean partitioning values of the drug for the two sets of RBCs are statistically different from each other. Within each individual population of RBCs, the partitioning of artemisinin at lower concentrations was found to be statistically different from that at higher concentrations. In excess of 92% of the drug added was accounted for by mass balance studies.
FIG. 3.
Partitioning of [14C]artemisinin in nonparasitized and parasitized (6% parasitemia) RBCs with eight different drug concentrations ranging from 0.88 to 3.52 μM. Triplicate samples with 33% hematocrit were incubated for 2 h at 37°C. The error bars indicate the relative standard deviation [relative standard deviation = (standard deviation/mean) × 100].
Temperature effect on partitioning.
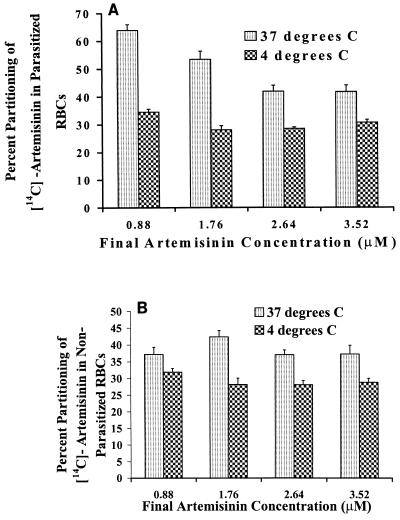
Nonparasitized RBCs incubated in the presence of four different drug concentrations ranging from 0.88 to 3.52 μM at 4°C for 2 h partitioned close to 30% of the drug (Fig. 4). Parasitized cells containing 6% parasitemia also partitioned 30 to 34% of the drug at 4°C, irrespective of the drug concentration used. Thus, a significant reduction in the partitioning of the drug into parasitized RBCs at 4°C in comparison to that seen at 37°C was observed (Fig. 4). A statistical evaluation of the mean partitioning values for all concentrations used, both for nonparasitized and for parasitized RBCs, revealed that they were significantly different from each other.
FIG. 4.
Partitioning of [14C]artemisinin in parasitized (6% parasitemia) (A) and nonparasitized (B) RBCs at 37 and 4°C with four different concentrations ranging from 0.88 to 3.52 μM. Each point is an average for seven replicates with 33% hematocrit and an incubation period of 2 h. The error bars indicate the relative standard deviation [relative standard deviation = (standard deviation/mean) × 100].
Competitive binding studies.
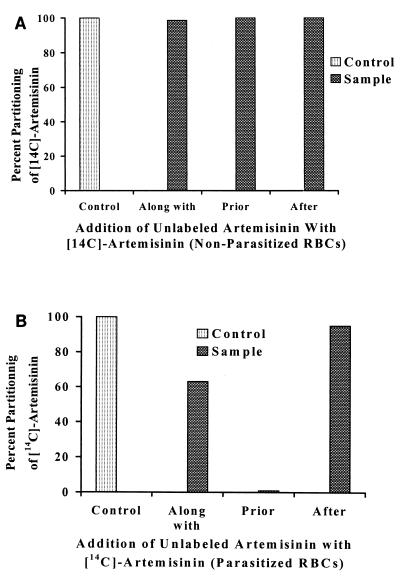
The presence of 0.88 μM (175 ng) unlabeled artemisinin was found to have a profound effect on the partitioning of the same amount of [14C]artemisinin into parasitized RBCs. (Note that these results were corrected for 100% parasitemia.) It was found that 104 ng of drug partitioned into parasitized RBCs in the absence of unlabeled artemisinin (control). This was an increase of 38 ng in comparison to the amount for nonparasitized RBCs, which partitioned 66 ng of the drug in the absence of unlabeled artemisinin. In the presence of unlabeled artemisinin added along with the radiolabeled drug, parasitized RBCs partitioned a total of 90 ng of [14C]artemisinin. This was an increase of only 24 ng in comparison to that for nonparasitized RBCs, which showed the partitioning of 66 ng of radiolabeled drug even in the presence of unlabeled artemisinin. Thus, while the presence of unlabeled artemisinin did not have any effect on the partitioning of [14C]artemisinin into nonparasitized cells, the pure parasitized RBCs showed close to 40% inhibition of the partitioning of [14C]artemisinin in the presence of the unlabeled drug (Fig. 5). When unlabeled artemisinin was added 1 h prior to as well as 1 h after the addition of [14C]artemisinin, nonparasitized cells still partitioned close to 66 ng of the radiolabeled drug. In contrast, parasitized cells did not show any partitioning of the radiolabeled drug in the presence of unlabeled artemisinin added 1 h prior to the addition of [14C]artemisinin. In the presence of 175 ng of unlabeled artemisinin added 1 h after the addition of the radiolabeled drug, partitioning of close to 38 ng of [14C]artemisinin was observed in parasitized RBCs, as before.
FIG. 5.
Effect of addition of 175 ng (0.88 μM) of unlabeled artemisinin on the partitioning of 0.88 μM [14C]artemisinin into both nonparasitized RBCs (A) and parasitized (7% parasitemia) RBCs (B). Triplicate samples with 33% hematocrit were prepared and incubated at 37°C for 2 h. The unlabeled artemisinin was added along with, 1 h prior to, and 1 h after the addition of [14C]artemisinin. The actual amount of drug partitioned was calculated, converted to percent drug partitioned, and plotted.
DISCUSSION
Uninfected RBCs did not show any selective partitioning and subsequent accumulation of the drug in comparison to the amount of drug present in the supernatant (data not shown). A constant 35 to 40% partitioning of the drug in nonparasitized cells was seen at 37°C, irrespective of the time of incubation or the drug concentration used. This is because of the hydrophobic drug’s inherent ability to diffuse passively across the RBC membrane, which is like any other biologic membrane: dynamic in nature and permeable to the passage of various solutes and drugs. In contrast, at 4°C, 28 to 30% of the drug partitioned into nonparasitized RBCs and a steady-state concentration was not reached. This may well be because at 4°C the rate of passive diffusion across the RBC membrane decreases due to the decreased lipid solubility of the drug, decreased viscosity, and other such considerations. The effect of the presence of unlabeled artemisinin on the partitioning of [14C]artemisinin between nonparasitized RBCs and the supernatant showed no inhibition of partitioning of the radiolabeled drug. This further supports the hypothesis that diffusion of artemisinin across the normal RBC membrane is passive in nature and, hence, is not subject to competition.
Parasitized RBCs are modified due to invasion of the malarial parasite (4, 9, 10, 23). With respect to the partitioning of artemisinin into parasitized RBCs, our studies provide further evidence that the process is carrier mediated. The partitioning of artemisinin into infected RBCs increased steadily to a level of 55 to 60% after 1 h. This is probably when all of the carrier proteins in the infected RBC membrane become saturated. Moreover, the fact that a steady decline in the percentage of drug partitioned into parasitized RBCs in comparison with the amount of drug in the supernatant was observed with increasing drug concentration is also an indication of carrier-mediated kinetics. The partitioning of artemisinin into parasitized cells at 4°C (a temperature at which only passive processes are known to be operative) was found to be almost half of that seen at 37°C. Parasitized RBCs therefore behaved as nonparasitized cells at 4°C, with the carrier-mediated component of the partition process inhibited and only passive diffusion across the RBC membrane operative.
The partitioning of [14C]artemisinin into parasitized RBCs was greatly affected in the presence of the same concentration of unlabeled artemisinin. An almost 40% reduction in the partitioning of the radiolabeled drug into parasitized RBCs was observed and can be explained by competition for the binding sites on the carrier molecules present in the modified parasitized RBCs. When unlabeled artemisinin was added 1 h prior to the addition of [14C]artemisinin, no partitioning of the radiolabeled drug into parasitized RBCs was detected. It is postulated that unlabeled artemisinin has saturated all of the binding sites on the parasitized RBC and that the 0.88 μM radiolabeled compound added after 1 h is insufficient to unbind the unlabeled drug, thus indicating an irreversible binding phenomenon at these low concentrations. Further evidence of irreversible binding was the inability of unlabeled artemisinin added 1 h after the addition of [14C]artemisinin to release any of the previously bound radiolabeled drug.
Acknowledgments
We acknowledge the National Institutes of Health for the financial support granted to carry out this work.
We also appreciate the contributions of Krishna Venkatesh, Barr Laboratories, and John Trott, NCNPR, University of Mississippi.
REFERENCES
- 1.Akompong, T., J. VanWye, N. Ghori, and K. Haldar. 1999. Artemisinin and its derivatives are transported by a vacuolar-network of Plasmodium falciparum and their antimalarial activities are additive with toxic sphingolipid analogues that block the network. Mol. Biochem. Parasitol. 101:71–79. [DOI] [PubMed] [Google Scholar]
- 2.Avery, M. A., J. D. Bonk, and J. Bupp. 1996. Radiolabeled antimalarials: synthesis of [14C]artemisinin. J. Labelled Compd. Radiopharm. 38:263–267. [Google Scholar]
- 3.Brossi, A., B. Venugoplalan, L. Dominguez Gerpe, H. J. Yeh, J. L. Flippen-Anderson, P. Buchs, X. D. Luo, W. Milhous, and W. Peters. 1988. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J. Med. Chem. 31:645–650. [DOI] [PubMed] [Google Scholar]
- 4.Cabantchik, Z. I. 1989. Altered membrane transport of malaria-infected erythrocytes: a possible pharmacological target. Blood 74:1464–1471. [PubMed] [Google Scholar]
- 5.De Vries, P. J., K. D. Tran, X. K. Nguyen, B. Le Nguyen, T. Y. Pham, D. D. Dao, C. J. Van Boxtel, and P. A. Kager. 1997. The pharmacokinetics of a single dose of artemisinin in patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 56:503–507. [DOI] [PubMed] [Google Scholar]
- 6.De Vries, P. J., and T. K. Dien. 1996. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818–836. [DOI] [PubMed] [Google Scholar]
- 7.Diribe, C. O., and D. C. Warhurst. 1985. A study of the uptake of chloroquine in malaria infected RBC. Biochem. Pharmacol. 34:3019–3027. [DOI] [PubMed] [Google Scholar]
- 8.Duc, D. D., P. J. De Vries, X. K. Nguyen, B. Le Nguyen, P. A. Kager, and C. J. van Boxtel. 1994. The pharmacokinetics of a single dose of artemisinin in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 51:785–790. [DOI] [PubMed] [Google Scholar]
- 9.Ginsburg, H. 1994. Transport pathways in the malaria-infected erythrocyte. Biochem. Pharmacol. 48:1847–1856. [DOI] [PubMed] [Google Scholar]
- 10.Ginsburg, H., and D. Stein. 1987. Biophysical analysis of novel transport pathways induced in red blood cell membranes. J. Membr. Biol. 96:1–10. [DOI] [PubMed] [Google Scholar]
- 11.Gu, H. M., D. C. Warhurst, and W. Peters. 1984. Uptake of [3H] dihydroartemisinin by RBC infected with Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 78:256–270. [DOI] [PubMed] [Google Scholar]
- 12.Hawley, S. R., P. G. Bray, P. M. O’Neill, B. K. Park, and S. A. Ward. 1996. The role of drug accumulation in 4-aminoquinoline antimalarial potency. Biochem. Pharmacol. 52:723–733. [DOI] [PubMed] [Google Scholar]
- 13.Hawley, S. R., P. G. Bray, B. K. Park, and S. A. Ward. 1996. Amodiaquine accumulation in Plasmodium falciparum as a possible explanation for its superior antimalarial activity over chloroquine. Mol. Biochem. Parasitol. 80:15–25. [DOI] [PubMed] [Google Scholar]
- 14.Hien, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603–608. [DOI] [PubMed] [Google Scholar]
- 15.Klayman, D. L. 1985. Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055. [DOI] [PubMed] [Google Scholar]
- 16.Krogstad, D. J., P. H. Schlesinger, and B. L. Herwaldt. 1988. Antimalarial agents: mechanism of chloroquine resistance. Antimicrob. Agents Chemother. 32:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loyevsky, M., S. Lytton, B. Mester, J. Libman, A. Shanzer, and Z. Cabantchik. 1993. The antimalarial action of desferal involves a direct access route to erythrocytic (Plasmodium falciparum) parasites. J. Clin. Investig. 91:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meshnick, S. R., and S. Kamchonwongpaisan. 1996. The mode of action of the antimalarial artemisinin and its derivatives. Gen. Pharmacol. 27:587–592. [DOI] [PubMed] [Google Scholar]
- 19.Meshnick, S. R. 1994. The mode of action of antimalarial endoperoxides. Trans. R. Soc. Trop. Med. Hyg. 88:31–32. [DOI] [PubMed] [Google Scholar]
- 20.Meshnick, S. R., T. E. Taylor, and S. Kamchonwongpaisan. 1996. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol. Rev. 60:301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meshnick, S. R., A. Thomas, A. Ranz, C. M. Xu, and H. Z. Pan. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181–189. [DOI] [PubMed] [Google Scholar]
- 22.Molyneux, E., T. E. Taylor, J. J. Wirima, and A. Borgstein. 1989. Clinical features and prognostic indicators in pediatric cerebral malaria: a study of 131 comatose Malawian children. Q. J. Med. 265:441–459. [PubMed] [Google Scholar]
- 23.Pasvol, G., B. Clough, and J. Carlsson. 1992. Malaria and the red cell membrane. Blood Rev. 6:183–192. [DOI] [PubMed] [Google Scholar]
- 24.San George, R. C., R. L. Nagel, and M. E. Fabry. 1984. On the mechanism for the red cell accumulation of mefloquine, an antimalarial drug. Biochim. Biophys. Acta 803:174–181. [DOI] [PubMed] [Google Scholar]
- 25.Trager, W., and J. B. Jenson. 1976. Human malaria parasites in continuous culture. Science 193:673–675. [DOI] [PubMed] [Google Scholar]
- 26.Ziffer, H., R. J. Highet, and D. L. Klayman. 1997. Artemisinin: an antimalarial from Artemisia annua L. Prog. Chem. Org. Natural Products 72:123–214. [DOI] [PubMed] [Google Scholar]