Abstract
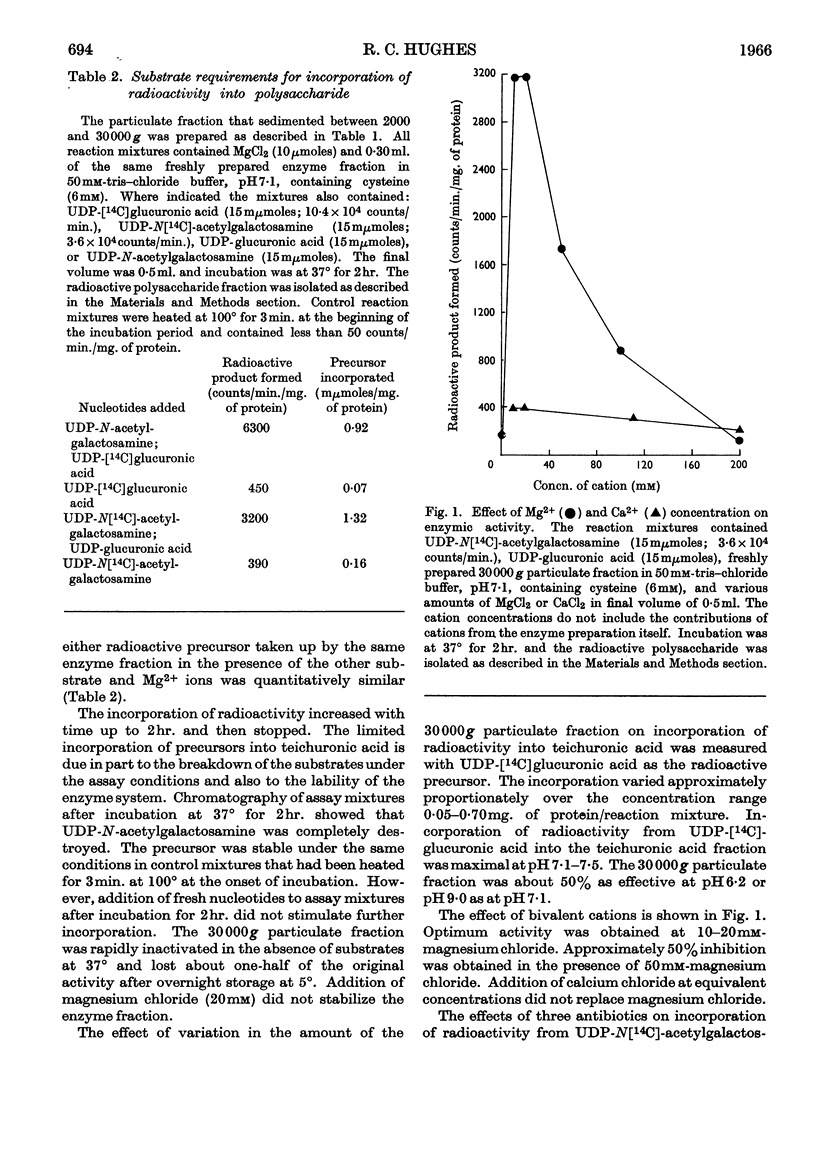
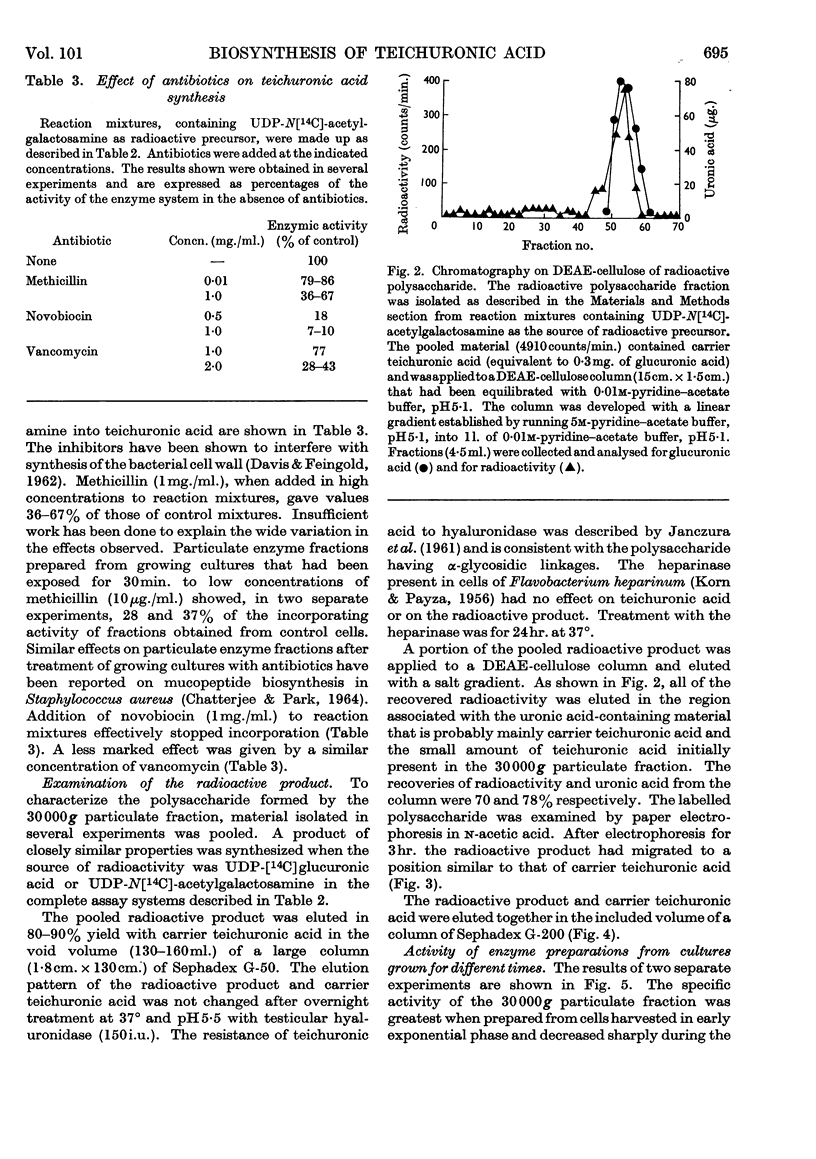
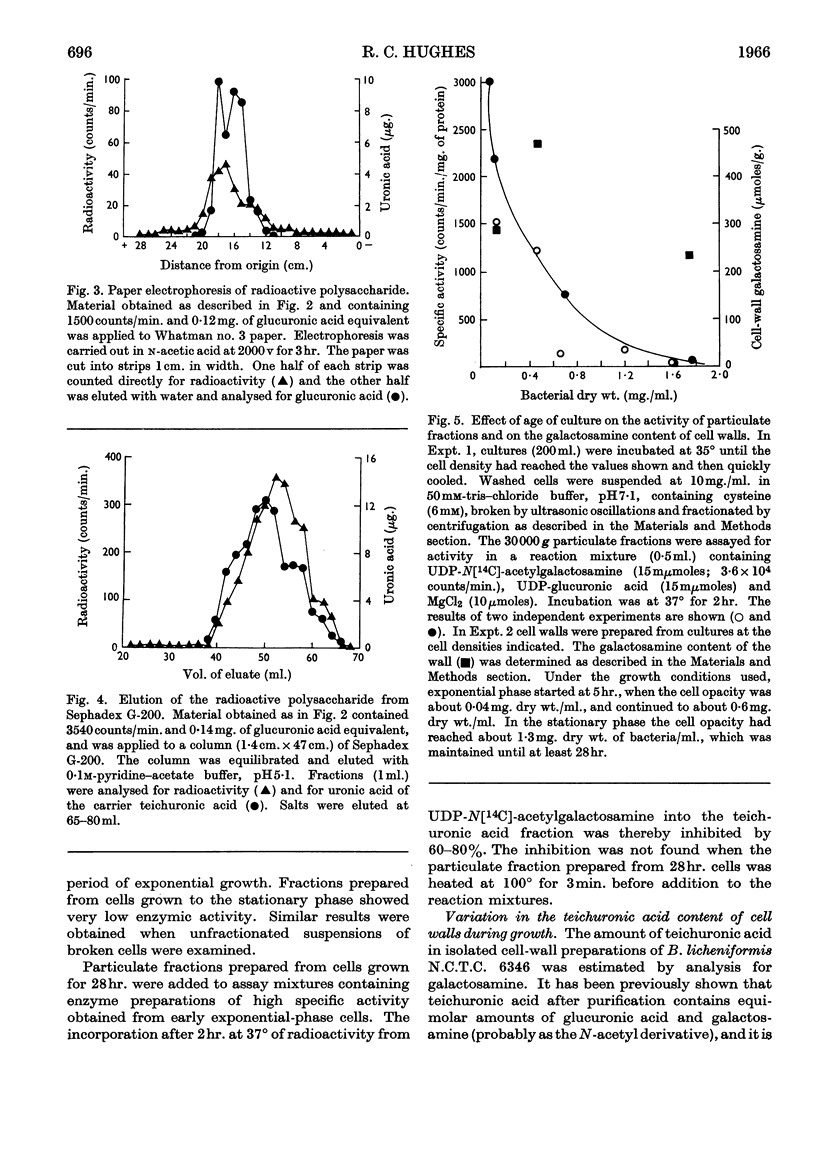
1. Particulate fractions prepared from disrupted cells of Bacillus licheniformis N.C.T.C. 6346 catalyse the uptake of radioactivity from UDP-[14C]glucuronic acid or UDP-N[14C]-acetylglucosamine. Maximal uptake requires the presence of both nucleotides and Mg2+ ions. The reaction is inhibited markedly by high concentrations of novobiocin and, to a certain extent, by vancomycin and by methicillin. 2. The radioactive product formed is resistant to Pronase and is soluble in 5% (w/v) trichloroacetic acid. It is of high molecular weight, from its behaviour on columns of Sephadex G-50 or G-200, and behaves during paper electrophoresis in n-acetic acid and chromatography on DEAE-cellulose in a manner similar to teichuronic acid. 3. Both teichuronic acid and the synthesized material are resistant to testicular hyaluronidase and to Flavobacterium heparinum heparinase. 4. The specific activity of suspensions of broken cells or of washed particulate fractions is greatest when they are prepared from exponentially growing cells. Fractions obtained from late exponential-phase or stationary-phase cells have very low activity. 5. The galactosamine content of B. licheniformis N.C.T.C. 6346 cell walls increases during the exponential phase and decreases during the stationary phase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., KALCKAR H. M., MAXWELL E. S., STROMINGER J. L. Enzymatic formation of uridine diphosphoglucuronic acid. J Biol Chem. 1957 Jan;224(1):79–90. [PubMed] [Google Scholar]
- CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A streptococcus. V. The uridine nucleotides of group A streptococcus. J Biol Chem. 1957 Oct;228(2):547–557. [PubMed] [Google Scholar]
- GLASER L. The synthesis of cellulose in cell-free extracts of Acetobacter xylinum. J Biol Chem. 1958 Jun;232(2):627–636. [PubMed] [Google Scholar]
- Hughes R. C. The isolation of structural components present in the cell wall of Bacillus licheniformis N.C.T.C. 6346. Biochem J. 1965 Sep;96(3):700–709. doi: 10.1042/bj0960700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCZURA E., PERKINS H. R., ROGERS H. J. Teichuronic acid: a mucopolysaccharide present in wall preparations from vegetative cells of Bacillus subtilis. Biochem J. 1961 Jul;80:82–93. doi: 10.1042/bj0800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D., PAYZA A. N. The degradation of heparin by bacterial enzymes. II. Acetone powder extracts. J Biol Chem. 1956 Dec;223(2):859–864. [PubMed] [Google Scholar]
- LINKER A., HOFFMAN P., MEYER K., SAMPSON P., KORN E. D. The formation of unsaturated disacharides from mucopoly-saccharides and their cleavage to alpha-keto acid by bacterial enzymes. J Biol Chem. 1960 Nov;235:3061–3065. [PubMed] [Google Scholar]
- LOWTHER D. A., ROGERS H. J. The role of glutamine in the biosynthesis of hyaluronate by streptococcal suspensions. Biochem J. 1956 Feb;62(2):304–314. doi: 10.1042/bj0620304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem. 1959 Sep;234:2343–2350. [PubMed] [Google Scholar]
- ROGERS H. J. The structure and function of hyaluronate. Biochem Soc Symp. 1961;20:51–79. [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]


