Abstract
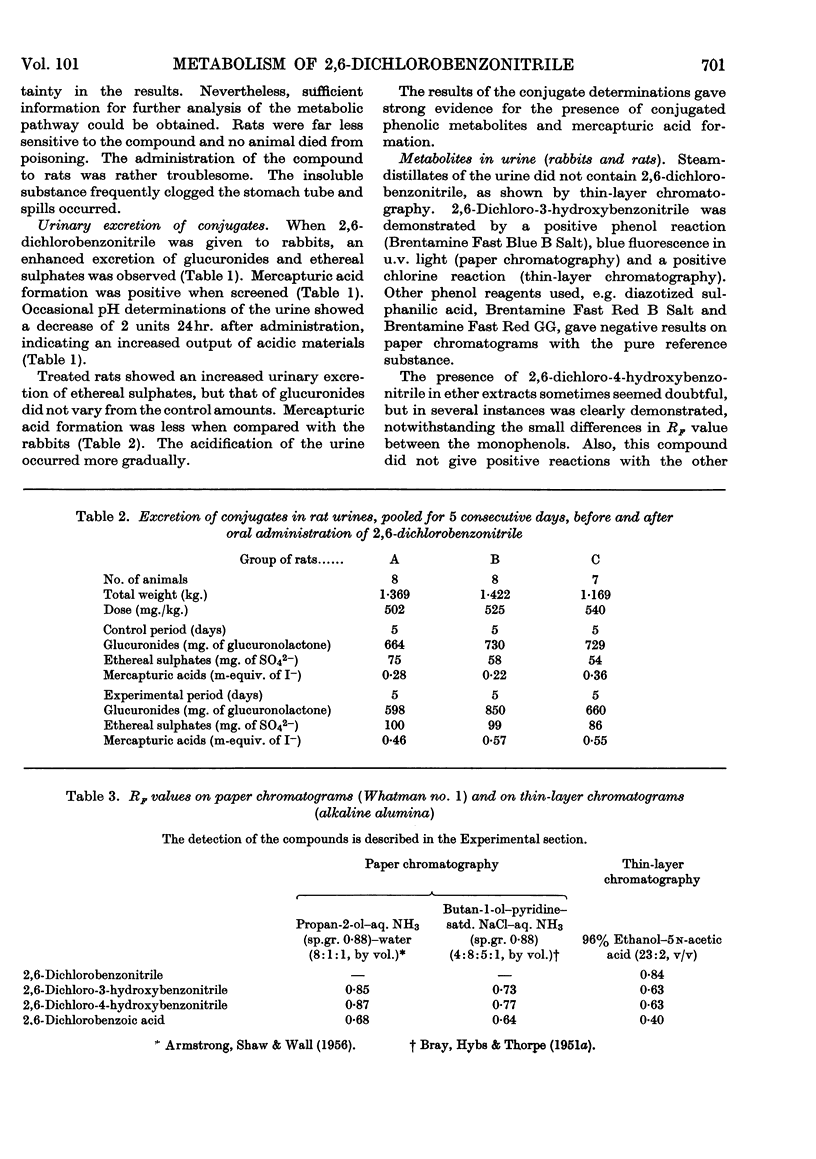
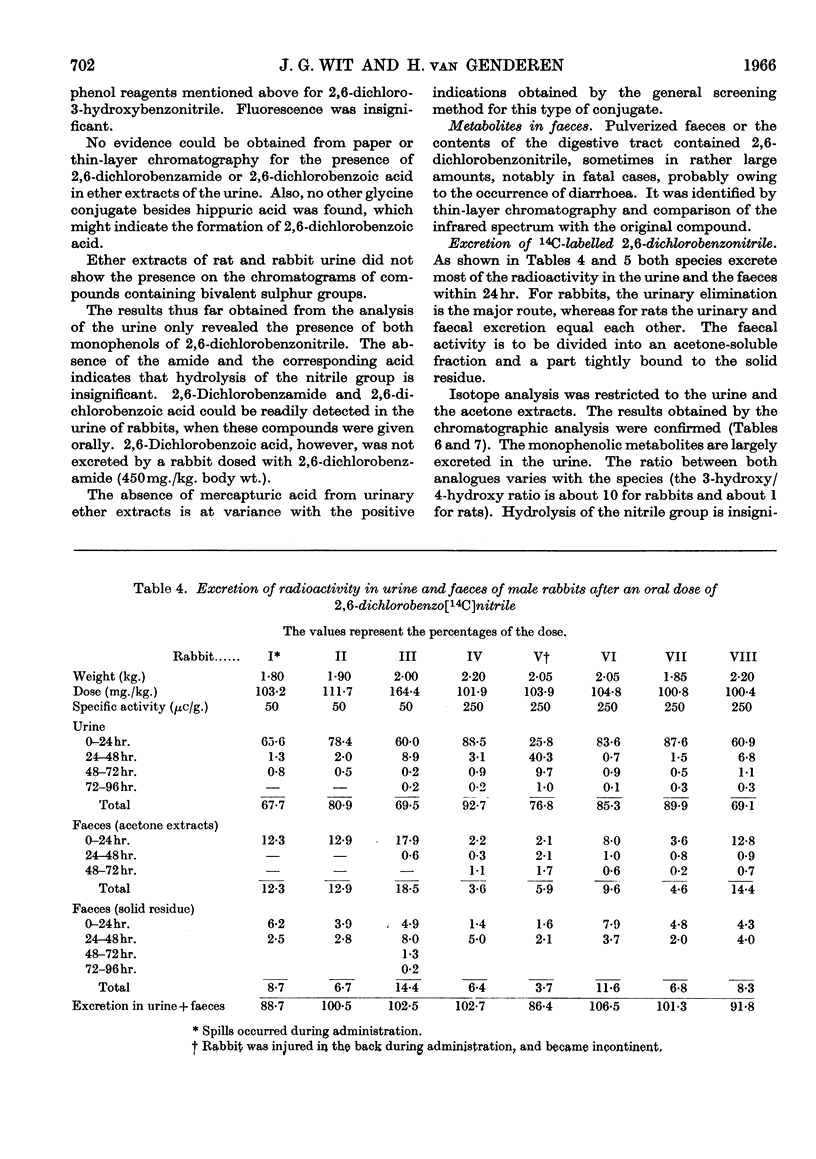
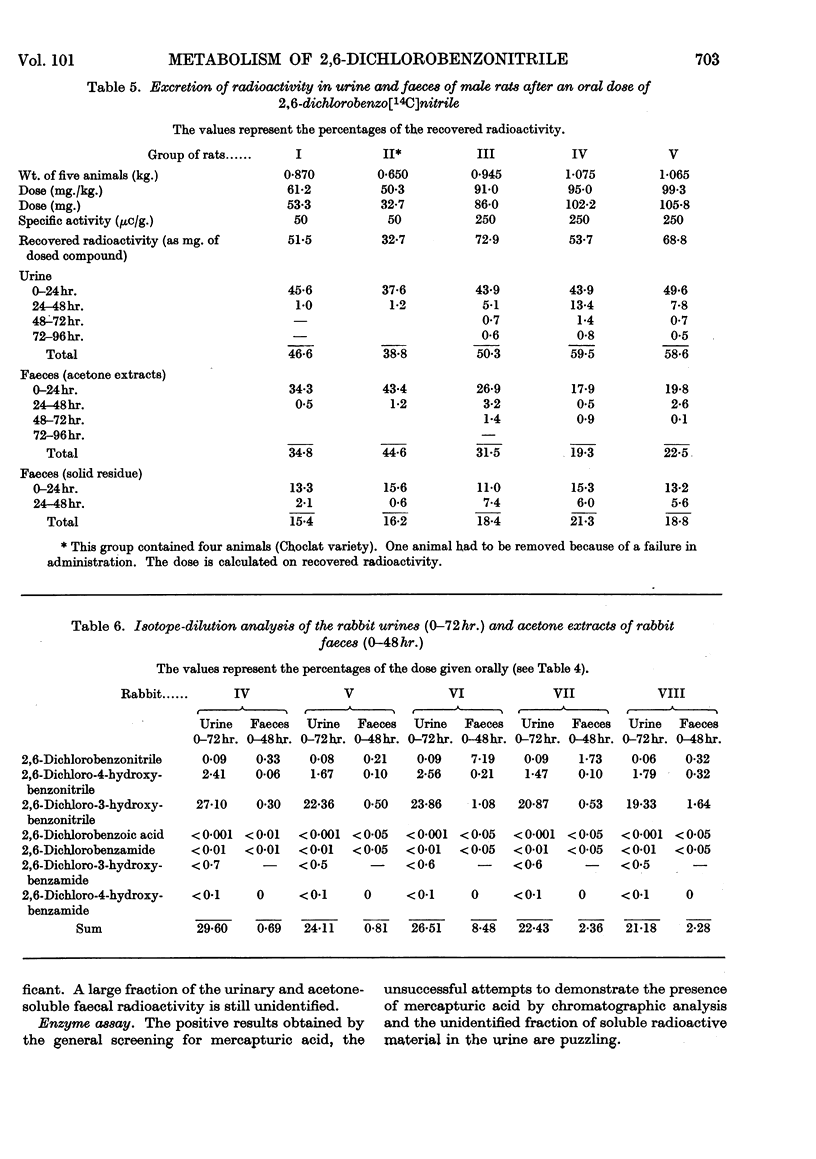
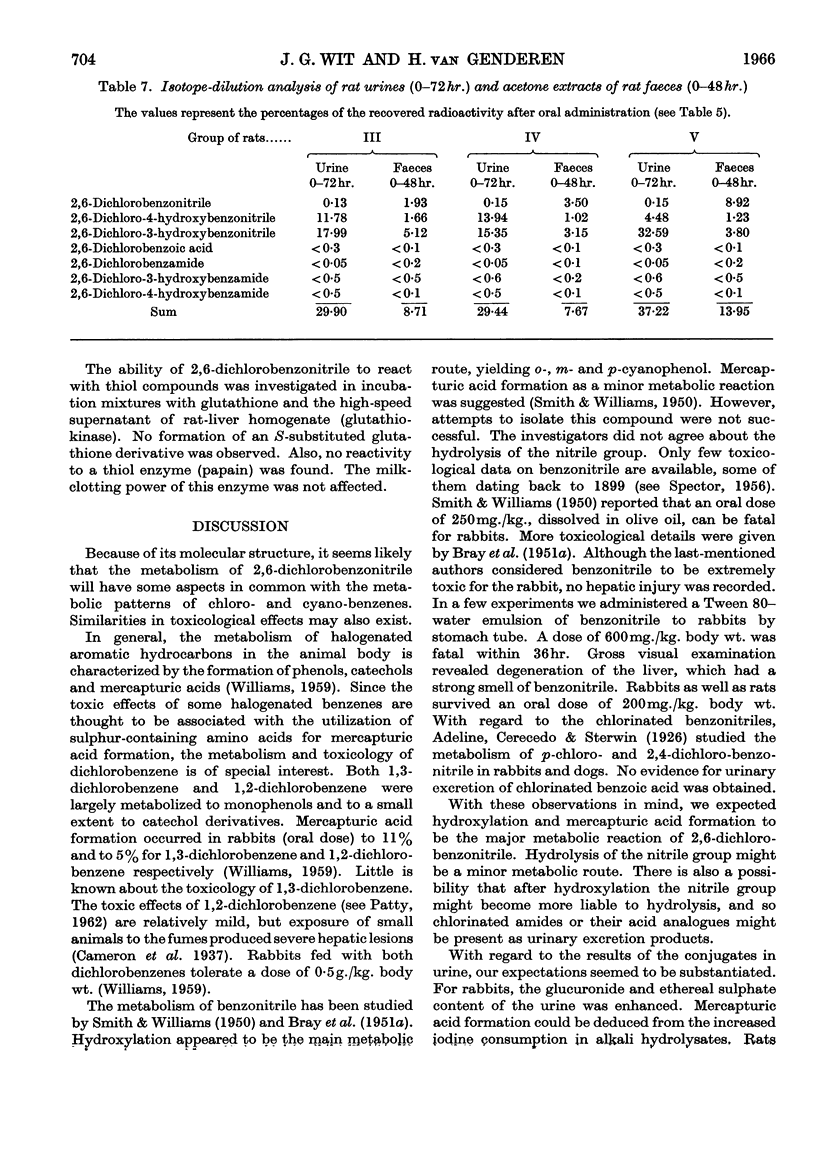
1. The metabolism of 2,6-dichlorobenzonitrile was studied in rabbits and rats. Oral administration caused an increased urinary excretion of glucuronides and ethereal sulphates. There was also an indication of mercapturic acid formation. 2,6-Dichloro-3-hydroxybenzonitrile and its 4-hydroxy analogue were identified as metabolites in the urine. A small amount of the unchanged substance was recovered from the faeces. 2. By using 2,6-dichlorobenzo[14C]nitrile the phenolic metabolites were determined quantitatively and some other possible metabolic routes were excluded. 3. Incubation of 2,6-dichlorobenzonitrile with enzyme preparations (papain and high-speed supernatant of rat-liver homogenate plus glutathione) gave no indications for a reaction with thiol compounds.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG M. D., SHAW K. N., WALL P. E. The phenolic acids of human urine; paper chromatography of phenolic acids. J Biol Chem. 1956 Jan;218(1):293–303. [PubMed] [Google Scholar]
- BRAY H. G., HYBS Z., THORPE W. V. Metabolism of derivatives of toluene; tolunitriles, benzonitrile and some related compounds. Biochem J. 1951 Feb;48(2):192–199. doi: 10.1042/bj0480192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAY H. G., THORPE W. V., VALLANCE D. K. The liberation of chloride ions from organic chloro compounds by tissue extracts. Biochem J. 1952 May;51(2):193–201. [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. H., Moss J. A., Rose J. A., Hathway D. E. The comparative metabolism of 2,6-dichlorothiobenzamide (Prefix) and 2,6-dichlorobenzonitrile in the dog and rat. Biochem J. 1966 Mar;98(3):770–781. doi: 10.1042/bj0980770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUTMAN A. C. LIQUID SCINTILLATION COUNTING OF BLOOD. Int J Appl Radiat Isot. 1965 Feb;16:65–70. doi: 10.1016/0020-708x(65)90149-3. [DOI] [PubMed] [Google Scholar]
- JONES E. R. H., HENBEST H. B., SMITH G. F., BENTLEY J. A. 3-indolylacetonitrile: a naturally occurring plant growth hormone. Nature. 1952 Mar 22;169(4299):485–487. doi: 10.1038/169485a0. [DOI] [PubMed] [Google Scholar]
- KNIGHT R. H., YOUNG L. Biochemical studies of toxic agents. 11. The occurrence of premercapturic acids. Biochem J. 1958 Sep;70(1):111–119. doi: 10.1042/bj0700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOPMAN H., DAAMS J. 2,6-Dichlorobenzonitrile: a new herbicide. Nature. 1960 Apr 2;186:89–90. doi: 10.1038/186089a0. [DOI] [PubMed] [Google Scholar]
- Milborrow B. V. The formation of 2,6-dichlorobenzonitrile from related compounds in plants. Biochem J. 1963 May;87(2):255–258. doi: 10.1042/bj0870255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER B., WILLIAMS R. T. Studies in detoxication: the influence of bromobenzene and cystine on the bromine content of the hair of rats. Biochem J. 1950 Apr;46(4):460–465. doi: 10.1042/bj0460460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. N., Williams R. T. Studies in detoxication. 31. The isolation of m- and p-cyanophenols as metabolites of cyanobenzene (benzonitrile) and the problem of the orientation of hydroxyl groups formed in vivo. Biochem J. 1950 Feb;46(2):243–248. doi: 10.1042/bj0460243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBERGER C. J., FIORESE F. F. Colorimetric method for hippuric acid. Clin Chem. 1963 Feb;9:91–96. [PubMed] [Google Scholar]
- Wit J. G., Van Genderen H. The monophenolic metabolites of the herbicide 2,6-dichlorobenzonitrile in animals as uncouplers of oxidative phosphorylation. Biochem J. 1966 Dec;101(3):707–710. doi: 10.1042/bj1010707. [DOI] [PMC free article] [PubMed] [Google Scholar]


