Abstract
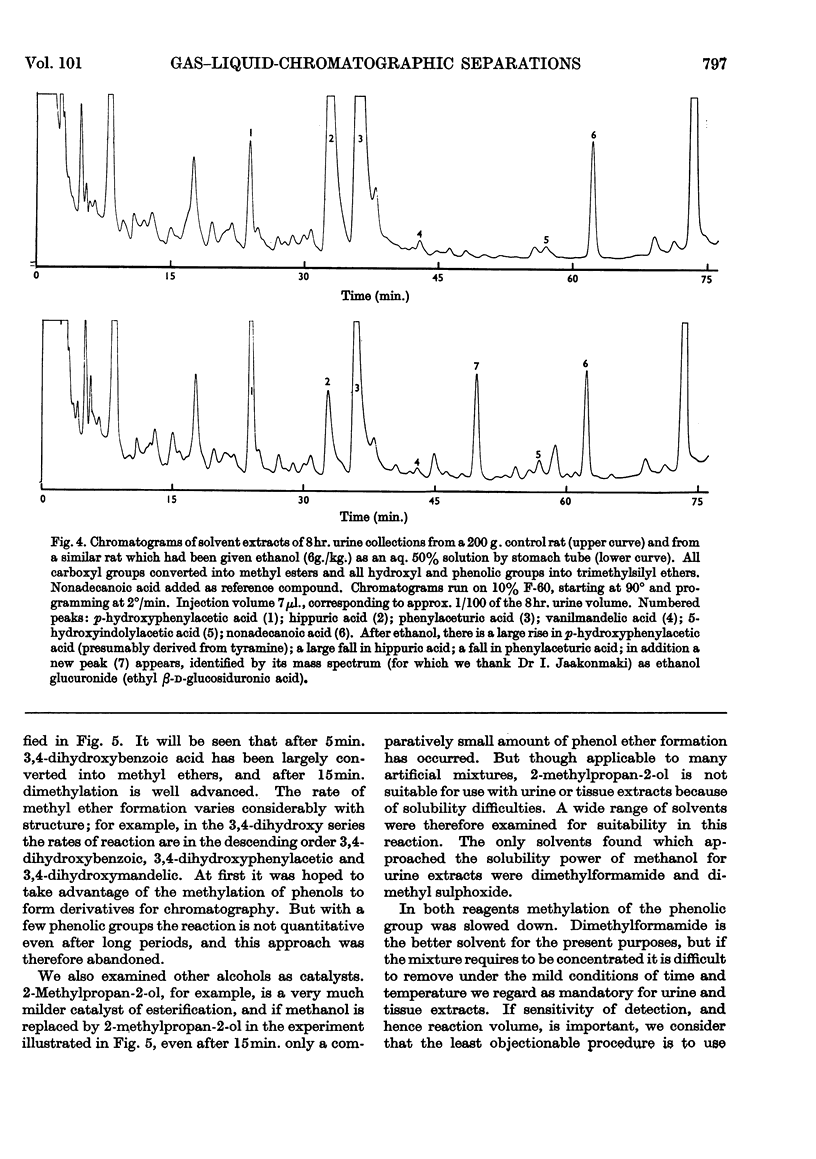
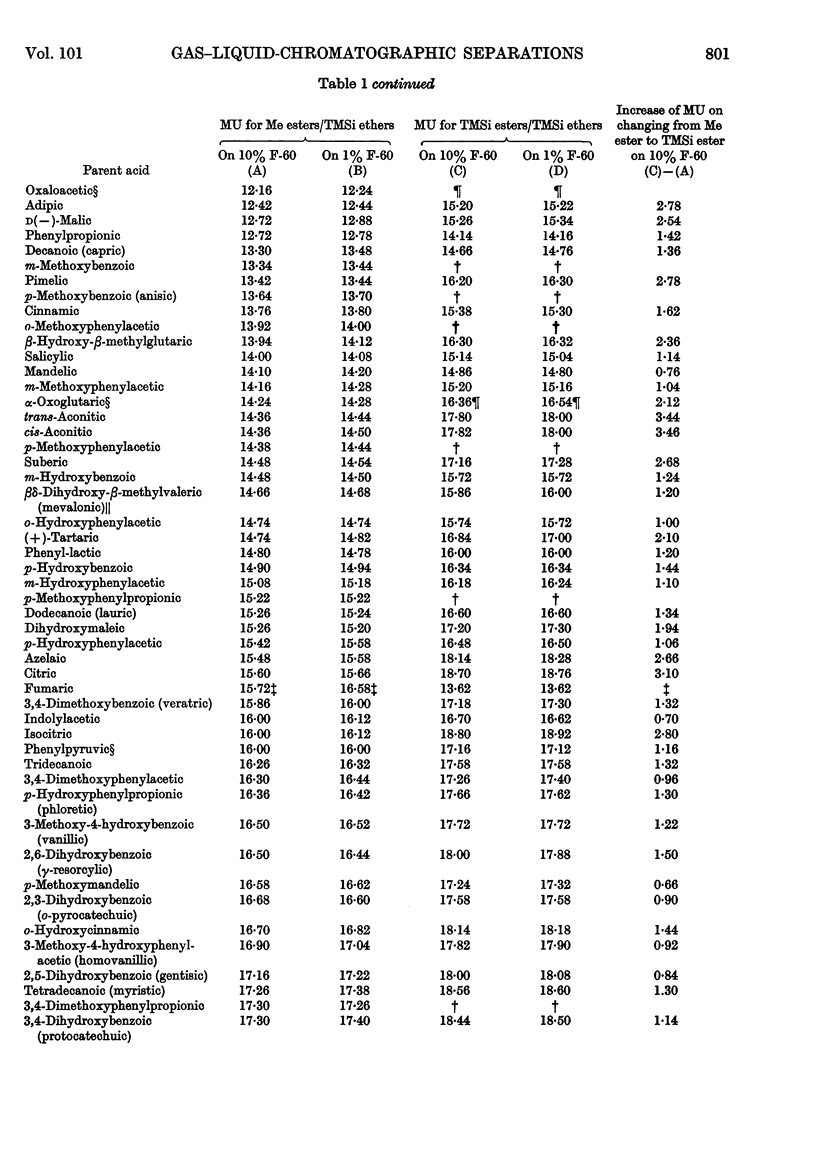
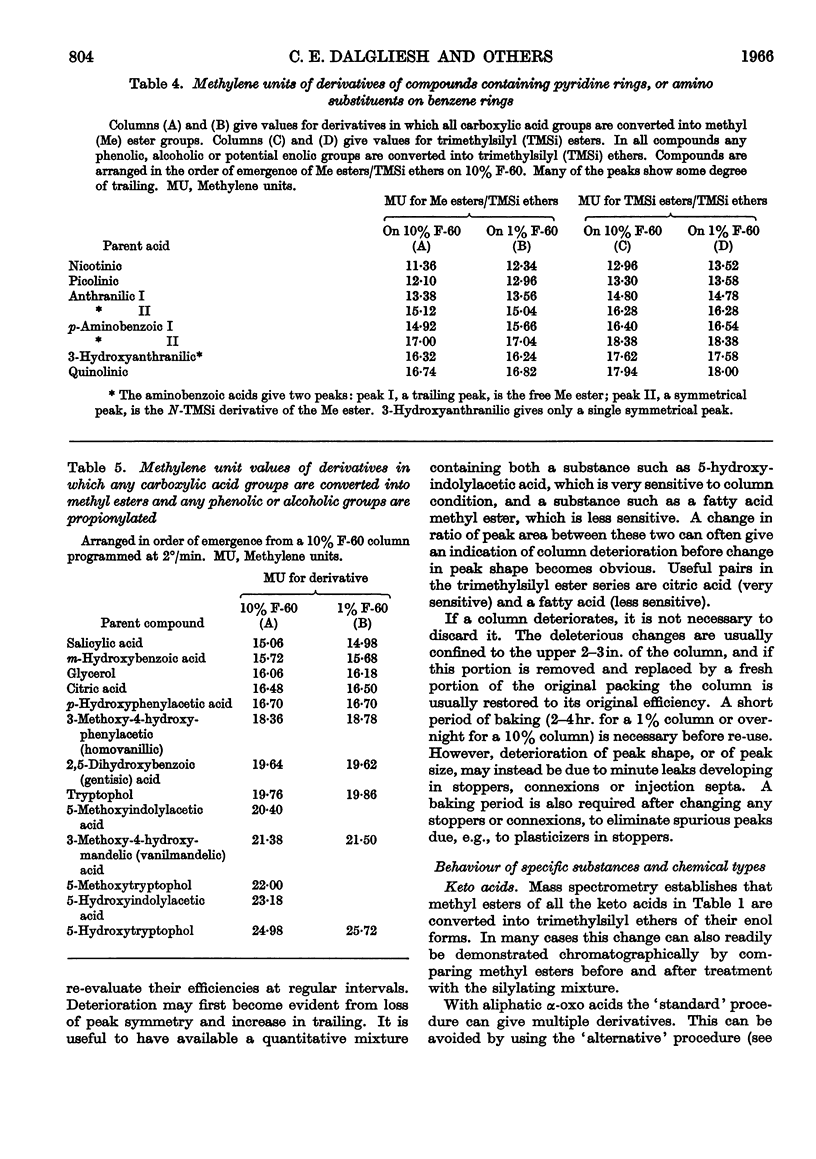
1. A gas–liquid-chromatographic procedure is described which permits separation and identification on the same chromatogram of a wide range of substances occurring in urine or tissue extracts. The method uses hydrogen flame ionization, which detects organic compounds whether free or conjugated with no requirement for specific reactive groups. 2. For chromatography, carboxyl groups are quantitatively converted into methyl esters or trimethylsilyl esters. Phenolic, alcoholic and potential enolic groups are converted into trimethylsilyl ethers. Separations are carried out on a 6ft. column of either 10% F-60 (a polysiloxane) or 1% F-60, temperature programming at 2°/min. being used over such part of the temperature range 30°–260° as is required. Propionyl derivatives of hydroxy compounds can also be used, but only on a non-quantitative basis. Derivatives and columns have been selected for optimum range of usefulness when large numbers of samples are examined by using automated gas chromatography. 3. The method is applicable to: fatty acids above butyric acid; di- and tri-carboxylic acids; hydroxy acids and keto acids; polyhydroxy and alicyclic compounds such as glycerol, inositol, quinic acid, shikimic acid, ascorbic acid and sugar alcohols; aromatic hydroxy and acidic compounds, both benzenoid and indolic; sesquiterpenes; steroids; glycine conjugates; mercapturic acids; glucuronides. It is not satisfactory for sulphate conjugates, iminazoles or polypeptides. 4. Methylene units provide an accurate and reproducible parameter for characterizing peak position. Methylene unit values are reported for a large variety of substances occurring in, or related to those occurring in, urine and tissue extracts. 5. The nature of derivatives was confirmed by combining gas chromatography with mass spectrometry. Combined gas chromatography–mass spectrometry gives a diagnostic tool of great power in the evaluation of metabolic patterns, and various uses are discussed.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capella P., Horning E. C. Separation and identification of derivatives of biologic amines by gas-liquid chromatography. Anal Chem. 1966 Feb;38(2):316–321. doi: 10.1021/ac60234a039. [DOI] [PubMed] [Google Scholar]
- ENEROTH P., HELLSTROEM K., RYHAGE R. IDENTIFICATION AND QUANTIFICATION OF NEUTRAL FECAL STEROIDS BY GAS-LIQUID CHROMATOGRAPHY AND MASS SPECTROMETRY: STUDIES OF HUMAN EXCRETION DURING TWO DIETARY REGIMENS. J Lipid Res. 1964 Apr;5:245–262. [PubMed] [Google Scholar]
- HORNING E. C., VANDENHEUVEL W. J., CREECH B. G. SEPARATION AND DETERMINATION OF STEROIDS BY GAS CHROMATOGRAPHY. Methods Biochem Anal. 1963;11:69–147. doi: 10.1002/9780470110294.ch2. [DOI] [PubMed] [Google Scholar]
- MAKITA M., WELLS W. W. Quantitative analysis of fecal bile acids by gas-liquid chromatography. Anal Biochem. 1963 Jun;5:523–530. doi: 10.1016/0003-2697(63)90072-1. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel W. J., Horning E. C. Analyse des steroïdes par chromatographie en phase gazeuse. Bull Soc Chim Biol (Paris) 1965;47(5):945–977. [PubMed] [Google Scholar]
- WILLIAMS C. M., LEONARD R. H. Microanalytical determination of dihydroxy aromatic acids by gas chromatography. Anal Biochem. 1963 Apr;5:362–366. doi: 10.1016/0003-2697(63)90089-7. [DOI] [PubMed] [Google Scholar]
- Williams C. M. Gas chromatography of the dihydroxybenzoic acids. Anal Biochem. 1965 May;11(2):224–229. doi: 10.1016/0003-2697(65)90009-6. [DOI] [PubMed] [Google Scholar]


