Abstract
To determine the efficacy of antibiotic catheter lock solution in preventing catheter-related infections, silicone catheters were tunneled and inserted into the jugular veins of 18 rabbits. The catheters were challenged with an intraluminal injection of 105 CFU of slime-producing Staphylococcus epidermidis in 0.1 ml of water. The catheters were maintained on heparin (100 IU/ml) flush for the first 3 days. On day 3, quantitative blood samples for culture were obtained from the catheters and ear veins, which documented catheter-related bacteremia, and the rabbits were randomized to have their catheters flushed as follows: five animals were continued on heparin (100 IU/ml), five animals received vancomycin (3 mg/ml) with heparin (100 IU/ml), and eight animals received 3 mg of minocycline per ml with 30 mg of EDTA per ml (M-EDTA). All animals were killed at day 7. Blood, catheters, jugular veins, and heart valves were cultured quantitatively. Animals maintained on heparin developed catheter-related colonization, bacteremia, septic phlebitis, and endocarditis. Vancomycin-heparin partially prevented catheter colonization, bacteremia, and phlebitis (P = 0.2). M-EDTA completely prevented catheter colonization, catheter-related bacteremia, and phlebitis in all of the animals (P < 0.01). Tricuspid endocarditis was equally prevented by vancomycin-heparin and M-EDTA (P ≤ 0.06). In conclusion, the M-EDTA catheter flush solution was highly efficacious in preventing catheter-related colonization, bacteremia, septic phlebitis, and endocarditis in rabbits.
Infections and thrombotic occlusions are the two most frequent complications from the use of central venous catheters (CVCs), and the two are pathogenetically related (21). Catheter-related bacteremia, which is often caused by staphylococcal organisms, is the leading cause of nosocomial bloodstream infections and is associated with high rates of morbidity and mortality in critically ill patients (9, 14, 17). Existing catheter flush solutions, such as heparin, are designed to prevent thrombotic occlusions but not catheter-related infections. Over the last decade, an antibiotic lock solution with or without anticoagulant (such as vancomycin-heparin) was used for the prevention and management of catheter-related bacteremia (1, 10, 11, 12, 22, 26). Concerns over the use of vancomycin flush solution were raised because of the potential for the development of organism resistance to this therapeutic agent (14, 28).
EDTA (disodium salt of ethylenediaminetetraacetic acid) is a calcium and iron chelator with anticoagulant activity and limited antistaphylococcal and anti-Candida activities (7, 23, 24). Minocycline is a tetracycline antibiotic with broad antistaphylococcal activity (31). We have previously demonstrated that minocycline and EDTA (M-EDTA) have highly synergistic activities in the decontamination of catheter surfaces when they are combined in a solution consisting of 3 mg of minocycline (Wyeth-Ayerst, Pearl River, N.Y.) and 30 mg of disodium EDTA (Endrate; Abbott Laboratories, North Chicago, Ill.) per ml of water and that M-EDTA is active against staphylococci, gram-negative bacilli, and Candida organisms that colonize polymers (I. Raad, R. Hachem, and R. Sherertz, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J57, 1994). With the animal model used previously (8), we compared the efficacy of M-EDTA to those of vancomycin-heparin and heparin alone in preventing catheter-related staphylococcal bacteremia and its complications (septic phlebitis and endocarditis.)
(This study was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, 15 to 18 September 1996, New Orleans, La.)
MATERIALS AND METHODS
Catheter placement.
Male New Zealand White rabbits (age, 2 to 3 months; weight, 2.5 to 3.5 kg) were used. The rabbits were housed in divided cages. Surgery was performed aseptically in an operating room. After general anesthesia was induced with 5% isoflurane, the rabbits were intubated and anesthetized with halothane (0.8 to 1.5%) in nitrous oxide-oxygen (2:1). A gas-sterilized 5 French silicone catheter (length, approximately 4 cm) was inserted into the right external jugular vein of each rabbit and was advanced anteriorly to the bifurcation of the internal and external jugular veins. A bulldog forceps was applied to the posterior facial vein. The vein was ligated above the clamp with 4.0 suture material. Another ligature was placed below the bulldog forceps but was not tied. The silastic catheter was inserted through a small incision in the vein above the clamp and was advanced so that the tip lay within the right anterior vena cava. The catheter was then secured in place with the loose ends of the ligatures. Catheter patency was tested by withdrawing a blood sample and flushing the catheter with heparinized saline.
The catheter was gripped in a Kelly hemostat and was then directed dorsally into a 2-cm pouch in the subcutaneous plane, as described by Hall et al. (8). A small incision was made, and the catheter was exteriorized through the opening and secured to the skin with a suture. Fluoroscopic monitoring was used during placement of the jugular catheter to visualize the placement of the tip of the catheter at the same level in the superior vena cavas of all the rabbits.
Organism inoculations.
Staphylococcus epidermidis isolated from a patient with catheter-related bacteremia at our center was grown overnight in Trypticase soy broth and was serially diluted in phosphate-buffered saline to achieve the desired inoculum of 105 CFU in 100 μl, which was injected into the CVC 4 h after surgical insertion.
Catheter treatment.
For the first 2 days of the study, the catheters of all the animals were flushed with heparin. On day 3, simultaneous blood samples for quantitative culture were drawn from the catheter and peripheral vein of each rabbit. Subsequently, the animals were randomly divided into three groups of rabbits according to a randomization scheme of with five, five, and eight animals in the three groups, respectively. Each animal in the first group of five animals was treated with heparin flush only. Each animal in the second group of five animals was treated with vancomycin plus heparin flush, and each animal in the third group of eight animals was treated with M-EDTA flush solution.
Heparin control group.
The patency of the catheter was maintained with daily 0.5-ml heparin (100 U/ml) flushes, with the heparin locked in the lumen until the 0.5-ml volume was flushed every 24 h on days 3, 4, 5, 6, and 7. The dead space of the catheter lumen was 0.35 ml.
Vancomycin-heparin group.
The catheters were flushed once daily with 0.5 ml of vancomycin-heparin solution (3 mg of vancomycin in 100 U of heparin). No precipitation was noted when vancomycin was mixed with heparin. The solution was locked in the lumen until the catheter was again flushed 24 h later on days 3, 4, 5, 6, and 7.
M-EDTA group.
Catheters were flushed once daily with 0.5 ml of M-EDTA (3 mg of minocycline and 30 mg of EDTA per ml of distilled water.) The solution was locked in the lumen until the catheter was again flushed 24 h later on days 3, 4, 5, 6, and 7.
Blood samples were collected from the CVCs and peripheral ear vein on days 3, 5, and 7 for quantitative culture. Pediatric Wampole isolator 1.5 microbial tubes (Wampole Laboratories, Cranbury, N.J.) were used for quantitative cultures. All rabbits were killed on day 7. The catheters were removed, and the proximal (subcutaneous) and distal (tip) segments (1 cm each) of the catheters were cultured by the scrape sonication technique (16, 27).
Tissue cultures and histopathology.
Postmortem bacteriological and histopathological examinations were performed on the jugular veins and heart valves of all the animals. All rabbits were killed on day 7. The jugular vein, the heart valve, and the lungs of each of these animals were removed aseptically, weighed, and transferred into sterile polyethylene bags with 5 ml of sterile saline and homogenized with a Stomacher 80 (A. J. Seward, UAC House, London, England). Samples were removed serially, diluted in 0.9% sterile saline, plated onto blood agar plates, incubated at 35°C, and examined at 24 h. The colonies were counted, and the numbers of CFU per gram of tissue were calculated. The microbiologists and pathologists were blinded as to which of the experimental groups the animals were randomized.
Definitions.
Catheter colonization was defined as ≥103 CFU of S. epidermidis in cultures of a catheter segment cultured by sonication (15, 27).
Catheter-related bacteremia was defined as the presence of S. epidermidis in a quantitative culture of blood drawn through the catheter at ≥10-fold the number of CFU per milliliter compared to the number in quantitative cultures of blood drawn from the peripheral ear vein (15, 26) or as colonization of a catheter segment with ≥103 CFU of S. epidermidis and cultures of peripheral vein blood concurrently positive for the same organism (15).
Septic phlebitis was defined as ≥103 CFU of S. epidermidis per g of jugular vein tissue in association with histopathologic evidence demonstrating septic phlebitis.
Infectious endocarditis was defined as ≥103 CFU of S. epidermidis per g of tissue from the heart valve in association with histopathologic evidence demonstrating evidence of endocarditis.
Statistical evaluation.
The outcome variables were the occurrence of positive cultures of blood from the CVC and peripheral ear vein, catheter colonization, catheter-related bacteremia, septic phlebitis, and endocarditis.
Testing of the significance of differences in outcomes by using discontinuous variables was done by the chi-square test or Fisher’s exact test. Continuous variables were tested by the Student t test. The cutoff level of significance was a P value of ≤0.05.
RESULTS
Catheter-related bacteremia.
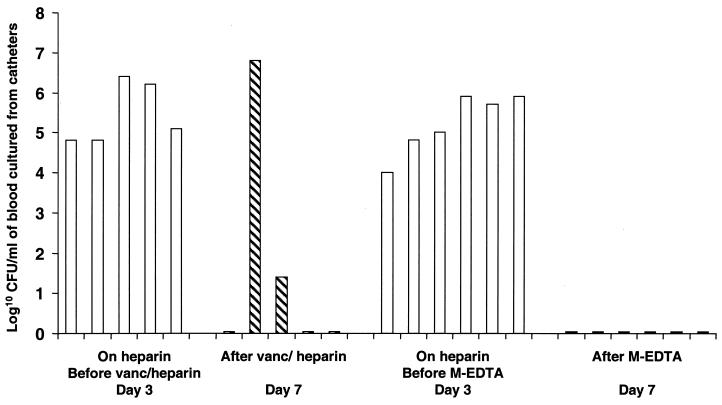
S. epidermidis was isolated on day 3 postinoculation from quantitative cultures of blood drawn from either the catheters or the peripheral ear veins of all 18 animals maintained on heparin. Peripheral blood from the five animals that remained on heparin flush continued to be positive by culture on days 5 and 7. In four of the animals on heparin flush, blood samples drawn from the catheter were positive by culture on days 5 and 7 (in one animal receiving heparin, the catheter was occluded). Prior to the initiation of treatment with vancomycin plus heparin, all five animals in that group developed catheter-related bacteremia, with the isolation of 6 × 104 to 2.6 × 106 CFU of S. epidermidis per ml of blood drawn through the CVC (Fig. 1). On day 7, two animals continued to have catheter-related bacteremia, whereas the other three had complete sterilization of the blood (Fig. 1) (P = 0.17). On day 3, prior to the initiation of M-EDTA treatment, six of the eight animals had evidence of catheter-related bacteremia. In the other two animals from which blood could not be drawn through the CVC because of luminal occlusion, peripheral blood samples were positive by culture on day 3. Following the initiation of M-EDTA treatment (on day 7), quantitative cultures of blood from the CVC became negative for all six animals whose peripheral blood samples were simultaneously negative (Fig. 1) (P < 0.001). In addition, peripheral blood samples from the two animals with occluded CVCs were negative by culture after the initiation of M-EDTA treatment (on days 5 and 7). Intraluminal catheter-related bacteremia persisted for animals receiving heparin (the control group). However, the catheter-related bloodstream infection was eliminated in 60% of those animals that received vancomycin plus heparin, and there was complete resolution of the catheter-related bacteremia in all of the animals that were placed on M-EDTA (Fig. 1).
FIG. 1.
Results of quantitative cultures of blood from catheter before and after intervention. Each bar represents the log10 CFU per milliliter of blood from an animal drawn on day 3 or 7. □, heparin group; ▧, vancomycin-heparin group; ▪, M-EDTA group. In addition to the six animals in the M-EDTA group, blood samples for culture could not be obtained from two additional animals with catheter occlusions; however, cultures of peripheral blood samples obtained from these animals on day 7 were negative. The flat lines on the x axis reflect negative cultures of samples from the animals.
It is noteworthy that peripheral blood from 80% (four of five) of the animals treated with heparin was positive by culture on days 3, 5, and 7. In addition, peripheral blood from 88% (seven of eight) of the animals randomized to the M-EDTA group had positive baseline cultures on day 3 prior to the initiation of M-EDTA flush. After the initiation of treatment with the M-EDTA catheter flush solution, peripheral blood from all eight animals in that group was negative by culture on days 5 and 7 (P < 0.0001). The consistency between positive cultures of peripheral blood and positive cultures of blood drawn through the catheter (as shown in Fig. 1) suggests that treatment with M-EDTA did result in the prevention and resolution of true bacteremias and that the effect was not related to a neutralization effect on the blood drawn through the catheter.
Catheter colonization.
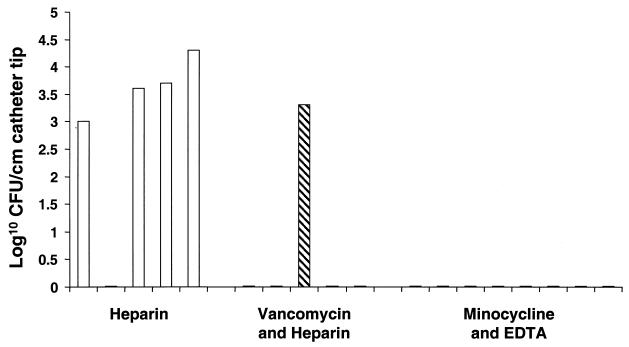
On day 7 levels of colonization ranging from 1 × 103 to 1.9 × 104 were detected in cultures of intravascular catheter tips from four of the five animals that were maintained on heparin, as determined by the scrape sonication quantitative catheter culture method and as shown in Fig. 2. Among the five animals that received vancomycin-heparin, colonization of the intravascular catheter tip segment was demonstrated in one of the animals, reflecting a partial decontamination of catheter surfaces compared to that for the heparin-treated control group (P = 0.2). No organisms were retrieved from the distal intravascular catheter tip segments of any of the eight animals that received M-EDTA, reflecting significant and complete decontamination of the catheter surfaces compared to the case for the heparin-treated control group (P < 0.01).
FIG. 2.
Results of quantitative culture of the catheter tip by the scrape sonication method. Each bar represents the results for a particular animal killed at day 7. □, heparin group; ▧, vancomycin-heparin group; ▪, M-EDTA group. The flat lines on the x axis reflect negative cultures of samples from the animals.
Septic phlebitis and endocarditis.
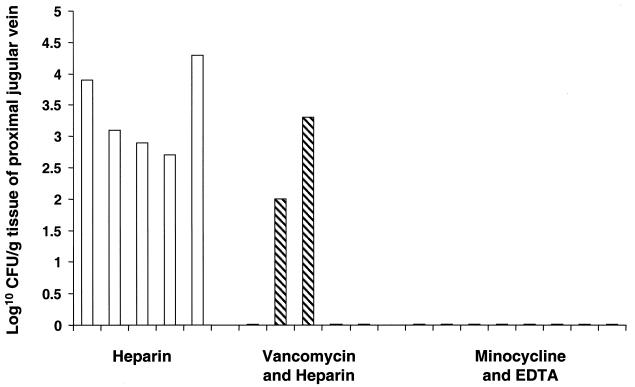
Septic phlebitis was demonstrated microbiologically and histopathologically in the jugular veins of all animals that were continued on heparin. As shown in Fig. 3, S. epidermidis was cultured from the proximal jugular vein at mean concentrations ranging from 5.3 × 102 to 1.8 × 104 CFU/g of proximal jugular vein tissue in all of the animals that received heparin (Fig. 3). In addition, the same organism was cultured from the distal jugular vein at a concentration of 2 × 102 to 2.5 × 105 CFU/g of distal vein tissue in all of the animals maintained on heparin. Vancomycin-heparin prevented septic phlebitis in three of the five animals (Fig. 3) (P = 0.17). However, in two other animals receiving vancomycin-heparin, S. epidermidis was cultured from the proximal jugular veins at concentrations of 2 × 102 and 3.4 × 103 CFU/g of jugular vein tissue, respectively. In all eight animals that received M-EDTA, the cultures of proximal and distal jugular vein tissues revealed no organisms, and hence, septic phlebitis was completely prevented (Fig. 3) (P < 0.001) For all of the animals in the heparin group and one of the animals in the vancomycin-heparin group, samples from different sites (blood through catheter, peripheral blood, catheter culture and jugular vein) of the same animal were persistently culture positive.
FIG. 3.
Results of quantitative culture of proximal jugular vein tissue. Each bar represents the results for a particular animal killed at day 7. □, heparin group; ▧, vancomycin-heparin group; ▪, M-EDTA group. The flat lines on the x axis reflect negative cultures of samples from the animals.
Catheter-related bacteremia attributed to luminal colonization resulted in infective endocarditis in four of the five animals that were continued on heparin. Use of the vancomycin-heparin flush solution resulted in the complete prevention of infective endocarditis in the five animals that received such a combination through the lumen of the catheter (P = 0.05). Similarly, use of M-EDTA resulted in the complete and statistically significant (P < 0.01) prevention of such complications when it was given through the lumen of the catheter.
DISCUSSION
In the animal model used in the present study, a large inoculum of S. epidermidis injected through the lumens of tunneled catheters in rabbits results in catheter-related bacteremia, septic phlebitis, and even endocarditis (18). S. epidermidis is the leading cause of catheter-related bloodstream infections in humans, but it is rarely associated with septic phlebitis and endocarditis (18). However, in this animal model, it behaves more like Staphylococcus aureus in the clinical setting, which is more commonly associated with septic phlebitis and endocarditis (4, 13). As an antibiotic lock solution, M-EDTA was highly efficacious in completely preventing catheter colonization, catheter-related bacteremia, catheter-related septic phlebitis, and endocarditis. Vancomycin-heparin antibiotic lock solution was shown to prevent catheter-related bacteremia and septic phlebitis in 60% of the animals, but it completely prevented endocarditis.
Several studies have demonstrated that antibiotic lock solutions are useful in the prevention and the adjunct treatment of tunneled CVC-related bloodstream infections (1, 10, 11, 12, 22, 26). Because staphylococci are the leading cause of catheter-related bloodstream infections, the combination of vancomycin and heparin has often been used (1, 10, 22, 26). At least three prospective, randomized studies have demonstrated that the combination of vancomycin and heparin is superior to heparin as an antibiotic lock solution in preventing catheter-related bacteremias attributed to vancomycin-susceptible organisms (1, 10, 26). One smaller prospective, randomized, controlled trial failed to show any benefit from the addition of vancomycin to heparin in decreasing the rate of catheter-related bacteremia (22). Despite its potential efficacy, the Centers for Disease Control and Prevention recommended against the use of vancomycin as a prophylactic flush agent in the prevention of catheter-related bloodstream infections since it is an independent risk factor for the acquisition of vancomycin-resistant enterococci (28). In addition, it has been shown that, as a glycopeptide antibiotic, vancomycin fails to eradicate microbial organisms embedded in the biofilm layer on the catheter surface (5, 6). The extracellular slime produced by S. epidermidis and made of exopolysaccharide has been shown to inhibit the antimicrobial activity of vancomycin (6). This may explain its limited activity in completely preventing catheter colonization, catheter-related bacteremia, and septic phlebitis in this animal model. Vancomycin’s antibacterial activity is limited to gram-positive bacteria, and in order to avoid superinfections with gram-negative bacilli, Henrickson et al. (10) used the triple combination of vancomycin, ciprofloxacin, and heparin. Use of this combination, although beneficial, would result in the furthering of resistance to another group of therapeutic agents such as the quinolones and would also increase the cost of the regimen.
EDTA is a known chelator with anticoagulant activity equivalent to that of heparin but with the advantage that it has a limited broad-spectrum inhibitory activity against methicillin-resistant staphylococci, gram-negative bacilli, and Candida (19, 23, 31). Minocycline has been shown to be highly active against methicillin-sensitive and -resistant staphylococci that cause catheter-related bloodstream infections (3). The activity of the combination of minocycline and EDTA in a suspension was shown to be synergistic against gram-negative bacilli (29, 30). More recently, we have shown that this combination is highly active against staphylococci, gram-negative bacilli such as Stenotrophomonas maltophilia, and even Candida embedded in the biofilms formed on catheter surfaces (20; Raad et al., 34th ICAAC).
The efficacy of the M-EDTA lock solution in our animal model could be related to several factors. The first is that the combination of minocycline and EDTA has been shown to be highly active against S. epidermidis organisms embedded in the biofilm environment (20). The second is that minocycline-EDTA is not inhibited by the extracellular slime but has been shown to disrupt the slimy biofilm layer (20). The mechanism of action of EDTA in inhibiting biofilm formation is possibly through its chelating activity on calcium, which is a component essential to the maintenance of the extracellular biofilm matrix (2, 20, 25). Once organisms become embedded in the biofilm layer, their resistance to antibiotics increases, and the combination of minocycline and EDTA is uniquely useful in disrupting the biofilm and synergistically eradicating organisms from the biofilm environment (5, 6, 20, 25). Recently, we have demonstrated that the combination of minocycline and EDTA is highly efficacious in preventing recurrent catheter-related bloodstream infections caused by S. epidermidis, S. aureus, and Enterobacter aerogenes in three patients (19). In a more recent prospective, randomized clinical trial, M-EDTA was shown to decrease the risk of catheter-related infection and colonization by ninefold in hemodialysis patients and had anticoagulant activity comparable to that of heparin (A. Bleyer, L. Mason, I. Raad, and R. Sherertz, 4th Decenniel Int. Conf. Nosocomial and Healthcare Associated Infect., CDC/SHEA, abstr. P-S1–31, p. 91, 2000).
Concerns of antibiotic resistance that arise from the use of vancomycin are related to the fact that vancomycin is a frontline therapeutic antimicrobial agent frequently used in the treatment of bloodstream infections. On the other hand, neither minocycline nor EDTA is used as a mainstream therapeutic agent, which would make them more fit for use as part of a prophylactic flush solution. In addition, when M-EDTA was used in humans at the same dose used in the rabbit model described here, it was not detected in the serum (19). The absence of M-EDTA from the systemic circulation would make the likelihood of the emergence of resistance less likely.
In conclusion, the combination of minocycline and EDTA flush solution was shown to be highly efficacious in preventing catheter-related colonization, bacteremia, septic phlebitis, and endocarditis in an animal model. Furthermore, given the fact that this combination has been shown to be disruptive to biofilms and to have broad-spectrum activity against staphylococci, gram-negative bacilli, and Candida, it should be further tested in a large, prospective, randomized, multicenter clinical trial.
REFERENCES
- 1.Carratala, J., J. Niubo, A. Fernandez-Sevilla, E. Juve, X. Castellsague, J. Berlanga, J. Linares, and F. Gudiol. 1999. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 43:2200–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, X., and P. S. Stewart. 2000. Biofilm removal caused by chemical treatments. Water Res. 34:4229–4233. [Google Scholar]
- 3.Darouiche, R. O., I. Raad, G. P. Bodey, and D. M. Musher. 1995. Antibiotic susceptibility of staphylococcal isolates from patients with vascular catheter-related bacteremia: potential role of the combination of minocycline and rifampin. Int. J. Antimicrob. Agents 6:31–36. [DOI] [PubMed] [Google Scholar]
- 4.Dugdale, D. C., and P. G. Ramsey. 1990. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am. J. Med. 89:137–141. [DOI] [PubMed] [Google Scholar]
- 5.Evans, R. C., and C. J. Holmes. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 31:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber, B. F., M. H. Kaplan, and A. G. Clogston. 1990. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J. Infect. Dis. 161:37–40. [DOI] [PubMed] [Google Scholar]
- 7.Gil, M. L., M. Casanova, and J. P. Martinez. 1994. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch. Microbiol. 161:489–494. [DOI] [PubMed] [Google Scholar]
- 8.Hall, L. L., O. H. DeLopez, A. Roberts, and F. A. Smith. 1974. Technical notes. A procedure for chronic intravenous catheterization in the rabbit. Lab. Anim. Sci. 24:79–83. [Google Scholar]
- 9.Heiselman, D. 1994. Nosocomial bloodstream infections in the critically ill. JAMA 272:1819–1820. [PubMed] [Google Scholar]
- 10.Henrickson, K. J., R. A. Axtell, S. M. Hoover, S. M. Kuhn, J. Pritchett, S. C. Kehl, and J. P. Klein. 2000. Prevention of central venous catheter-related infections and the thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double-blind trial. J. Clin. Oncol. 18:1269–1278. [DOI] [PubMed] [Google Scholar]
- 11.John, D. C., F. L. Johnson, and S. Goldman. 1994. Preliminary results treating persistent central venous catheter-related infections with the antibiotic lock technique in pediatric patients. Pediatr. Infect. Dis. J. 13:930–931. [DOI] [PubMed] [Google Scholar]
- 12.Krzywda, E. A., D. A. Andris, C. E. Edmiston, and E. J. Quebbeman. 1995. Treatment of Hickman catheter sepsis using antibiotic lock technique. Infect. Control Hosp. Epidemiol. 16:596–598. [DOI] [PubMed] [Google Scholar]
- 13.Malanoski, G. J., M. H. Samore, A. Pefanis, and A. W. Karchmer. 1995. Staphylococcus aureus catheter associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch. Intern. Med. 155:1161–1166. [DOI] [PubMed] [Google Scholar]
- 14.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391–402. [DOI] [PubMed] [Google Scholar]
- 15.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O’Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis 32:1249–1272. [DOI] [PubMed] [Google Scholar]
- 16.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittet, D., D. Tarara, and R. P. Wenzel. 1994. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601. [DOI] [PubMed] [Google Scholar]
- 18.Raad, I., A. Alrahwan, and K. Rolston. 1998. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin. Infect. Dis. 26:1182–1187. [DOI] [PubMed] [Google Scholar]
- 19.Raad, I., A. Buzaid, J. Rhyne, R. Hachem, R. Darouiche, H. Safar, M. Albitar, and R. J. Sherertz. 1997. Minocycline and ethylenediaminetetraacetate for the prevention of recurrent vascular catheter-related infections. Clin. Infect. Dis. 25:149–151. [DOI] [PubMed] [Google Scholar]
- 20.Raad, I., and R. J. Sherertz. November1994. M-EDTA pharmaceutical preparations and uses thereof. U.S. patent 5, 362,754.
- 21.Raad, I. I., M. Luna, S.-A. M. Khalil, J. W. Costerton, C. L. Lam, and G. P. Bodey. 1994. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA 271:1014–1016. [PubMed] [Google Scholar]
- 22.Rackoff, W. R., M. Weiman, D. Jakobowski, R. Hirschl, V. Stallings, J. Bilodeau, P. Danz, L. Bell, and B. Lange. 1995. A randomized, controlled trial of the efficacy of a heparin and vancomycin solution in preventing central venous catheter-related infections in children. J. Pediatr. 127:147–151. [DOI] [PubMed] [Google Scholar]
- 23.Reardon, D. M., B. Warner, and E. A. Trowbridge. 1991. EDTA, the traditional anticoagulant of haematology: with increased automation is it time for review? Med. Lab. Sci. 48:72–75. [PubMed] [Google Scholar]
- 24.Root, J. L., O. R. McIntyre, N. J. Jacobs, and C. P. Daghlian. 1988. Inhibitory effect of disodium EDTA upon the growth of Staphylococcus epidermidis in vitro: relation to infection prophylaxis of Hickman catheters. Antimicrob. Agents Chemother. 32:1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose, R. K. 2000. The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim. Biophys. Acta 1475:76–82. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz, C., K. J. Henrickson, K. Roghmann, and K. Powell. 1990. Prevention of bacteremia attributed to luminal colonization of tunneled central venous catheters with vancomycin-susceptible organisms. J. Clin. Oncol. 8:1591–1597. [DOI] [PubMed] [Google Scholar]
- 27.Sherertz, R. J., I. Raad, A. Belani, L. Koo, and K. Rand. 1990. Sonication vascular catheter cultures: a 3-year experience in a clinical microbiology laboratory. J. Clin. Microbiol. 28:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spafford, P. S., R. A. Sinkin, C. Cox, L. Reubens, and K. R. Powell. 1994. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. 44:1–13. [PubMed] [Google Scholar]
- 29.Wooley, R. E., M. Jones, J. P. Gilbert, and E. B. Shotts, Jr. 1983. In vitro action of combinations of antimicrobial agents and EDTA-tromethamine on Pseudomonas aeruginosa. Am. J. Vet. Res. 44:1521–1524. [PubMed] [Google Scholar]
- 30.Wooley, R. E., M. S. Jones, J. P. Gilbert, and E. B. Shotts, Jr. 1983. In vitro action of combinations of antimicrobial agents and EDTA-tromethamine on Escherichia coli. Am. J. Vet. Res. 44:1154–1158. [PubMed] [Google Scholar]
- 31.Yuk, J. H., M. C. Dignani, R. L. Harris, M. W. Bradshaw, and T. W. Williams. 1991. Minocycline as an alternative antistaphycococcal agent. Rev. Infect. Dis. 13:1023–1024. [DOI] [PubMed] [Google Scholar]