Abstract
1. Hb-FI, with the previously proposed structure αA2γFγAcF, would be an exception to the general hypothesis that haemoglobins are in mobile equilibrium with their sub-units at all pH values. However, studies presented in this paper suggest that this is not so. 2. Gel-filtration and sedimentation analyses show that Hb-FI dissociates at acid pH and gives only the usual types of hybrids in recombination experiments. When self-hybridized, Hb-FI is the main haemoglobin species re-formed, although small but increasing amounts of Hb-FII appear on prolonged exposure to acid. 3. Exchange experiments with isotopically labelled Hb-FII and unlabelled Hb-FI show no exchange of sub-units at neutral pH or after brief exposure to acid pH. Under equilibrium conditions at acid pH non-αA-chains do not exchange, although αA-chains equilibrate completely between the two species. 4. These results indicate that Hb-FI does not contain γF-chains and its possible structure is discussed on this basis. Since the dissociation properties of Hb-FI are not markedly different from those of Hb-A or Hb-FII it is concluded that Hb-FI, like other haemoglobins, is an equilibrium system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., THOMPSON T. E. DETERMINATION OF STOICHIOMETRY AND EQUILIBRIUM CONSTANTS FOR REVERSIBLY ASSOCIATING SYSTEMS BY MOLECULAR SIEVE CHROMATOGRAPHY. Proc Natl Acad Sci U S A. 1965 Feb;53:342–349. doi: 10.1073/pnas.53.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCCI E., FRONTICELLI C., DANCE N., SHOOTER E. M. FURTHER STUDIES ON THE STRUCTURE AND PROPERTIES OF HUMAN HAEMOGLOBINS MODIFIED BY DIGESTION WITH THE CARBOXYPEPTIDASES. J Mol Biol. 1965 Jan;11:109–115. doi: 10.1016/s0022-2836(65)80176-0. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Macduff G. Subunit exchange and ligand binding: a new hypothesis for the mechanism of oxygenation of hemoglobin. Proc Natl Acad Sci U S A. 1965 Aug;54(2):535–542. doi: 10.1073/pnas.54.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCE N., HUEHNS E. R., BEAVEN G. H. The abnormal haemoglobins in haemoglobin-H disease. Biochem J. 1963 May;87:240–248. doi: 10.1042/bj0870240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMMACK D. B., HUEHNS E. R., SHOOTER E. M., GERALD P. S. Identification of the abnormal polypeptide chain of hemoglobin G-Ib. J Mol Biol. 1960 Dec;2:372–378. doi: 10.1016/s0022-2836(60)80048-4. [DOI] [PubMed] [Google Scholar]
- GRATZER W. B., BEAVEN G. H. Transparent starch gels: preparation, optical properties and application to haemoglobin characterisation. Clin Chim Acta. 1960 Jul;5:577–582. doi: 10.1016/0009-8981(60)90071-1. [DOI] [PubMed] [Google Scholar]
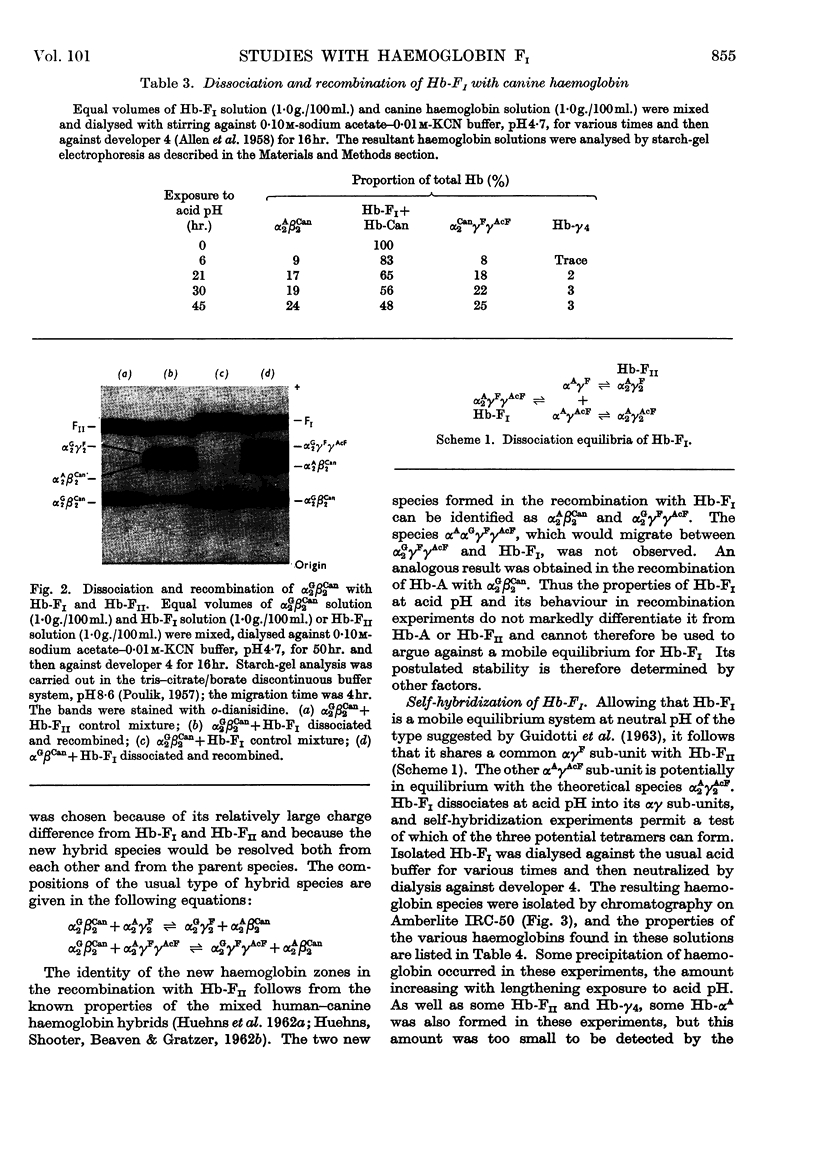
- GUIDOTTI G., KONIGSBERG W., CRAIG L. C. ON THE DISSOCIATION OF NORMAL ADULT HUMAN HEMOGLOBIN. Proc Natl Acad Sci U S A. 1963 Oct;50:774–782. doi: 10.1073/pnas.50.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQUIST W. R., SCHROEDER W. A. PROPERTIES AND PARTIAL CHARACTERIZATION OF ADULT HUMAN HEMOGLOBIN A1C. Biochim Biophys Acta. 1964 Mar 16;82:639–641. doi: 10.1016/0304-4165(64)90466-0. [DOI] [PubMed] [Google Scholar]
- HUEHNS E. R., DANCE N., JACOBS M., BEAVEN G. H., SHOOTER E. M. ISOLATION AND PROPERTIES OF THE BETA-A- AND GAMMA-F-CHAIN SUBUNITS FROM NORMAL ADULT AND FOETAL HAEMOGLOBINS. J Mol Biol. 1965 May;12:215–224. doi: 10.1016/s0022-2836(65)80295-9. [DOI] [PubMed] [Google Scholar]
- HUEHNS E. R., DANCE N., SHOOTER E. M., BEAVEN G. H., GRATZER W. B. Some properties of the alpha2 and gamma2 subunits of foetal haemoglobin. J Mol Biol. 1962 May;4:329–337. doi: 10.1016/s0022-2836(62)80013-8. [DOI] [PubMed] [Google Scholar]
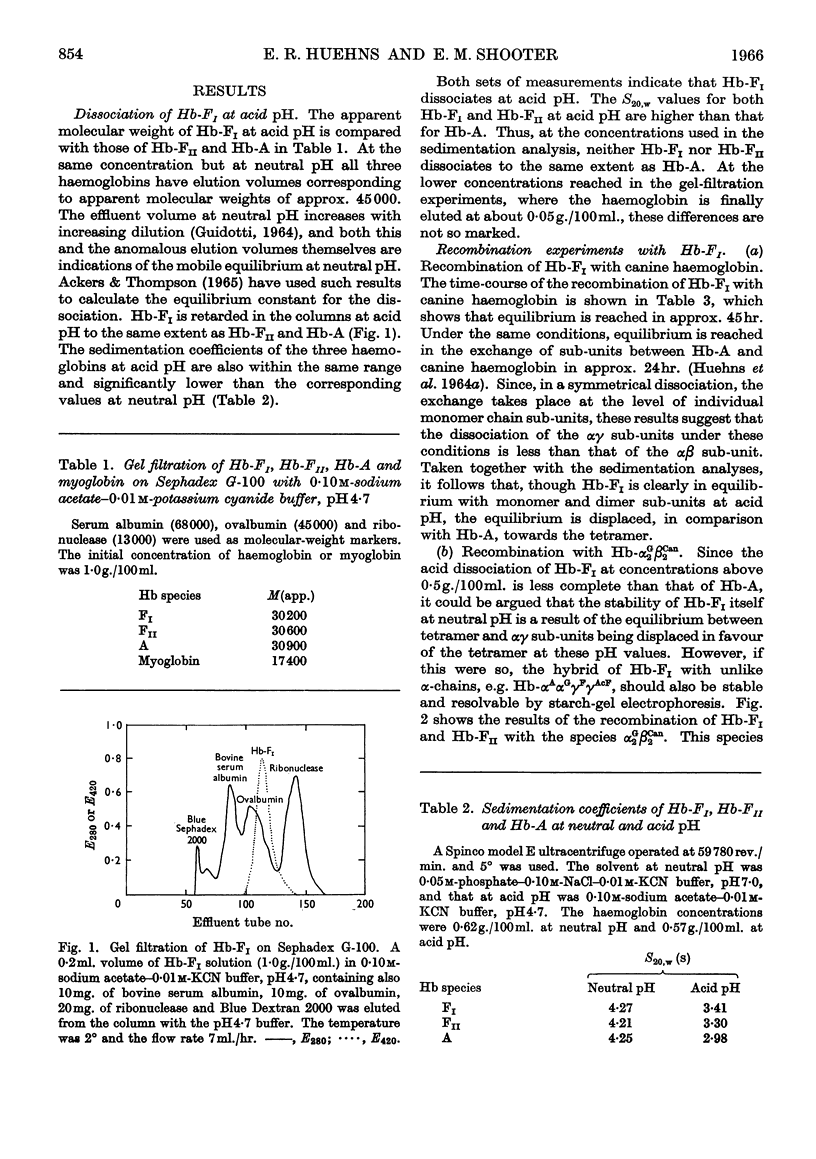
- HUEHNS E. R., SHOOTER E. M., BEAVEN G. H. On the recombination of canine and human haemoglobins. J Mol Biol. 1962 May;4:323–328. doi: 10.1016/s0022-2836(62)80012-6. [DOI] [PubMed] [Google Scholar]
- Huehns E. R., Beaven G. H., Stevens B. L. Recombination studies on haemoglobins at neutral pH. Biochem J. 1964 Aug;92(2):440–444. doi: 10.1042/bj0920440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehns E. R. Further studies on the isolation and properties of alpha-chain sub-units of haemoglobin. Biochem J. 1966 Dec;101(3):843–851. doi: 10.1042/bj1010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehns E. R., Shooter E. M., Beaven G. H. The time course of the recombination of human adult and canine haemoglobins. Biochem J. 1964 May;91(2):331–334. doi: 10.1042/bj0910331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm O., Polson A. The determination of diffusion constants of proteins by a refractometric method. Biochem J. 1936 Mar;30(3):528–541. doi: 10.1042/bj0300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- SCHROEDER W. A., CUA J. T., MATSUDA G., FENNINGER W. D. Hemoglobin F1, an acetyl-containing hemoglobin. Biochim Biophys Acta. 1962 Oct 8;63:532–534. doi: 10.1016/0006-3002(62)90125-7. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M., STEINBERG D., LOGAN J. Liquid scintillation counting of C14- and H3-labeled amino acids and proteins. Science. 1957 Sep 6;126(3271):446–447. doi: 10.1126/science.126.3271.446-a. [DOI] [PubMed] [Google Scholar]
- WINTERHALTER K. H., HUEHNS E. R. PREPARATIONS, PROPERTIES, AND SPECIFIC RECOMBINATION OF ALPHA-BETA-GLOBIN SUBUNITS. J Biol Chem. 1964 Nov;239:3699–3705. [PubMed] [Google Scholar]