Abstract
Antiviral activity of sulfated sialyl lipid (NMSO3) against human rotavirus (RV) was examined in vitro and in vivo. NMSO3 inhibited the replication of four major serotypes (G1 to G4) of human rotavirus with a low 50% effective concentration of 1 to 5 μg/ml and 50% cytotoxic concentration of 153 μg/ml when determined by plaque assays with MA104 cells. Exposure of NMSO3 to HCl (pH 2.0) for 30 min exhibited no loss of anti-RV activity. Time-of-addition experiments revealed that NMSO3 inhibited the adsorption of four serotypes of RV to MA104 cells. Furthermore, an assay of virus binding with radiolabeled RVs revealed that NMSO3 inhibited the binding of virus to MA104 cells, suggesting that NMSO3 may bind to VP4 and/or VP7. Prophylactic oral administration of NMSO3 (10 μg three times per day, 4 days) to five suckling mice starting 30 min before inoculation of MO strain (3 × 106 PFU/mouse) prevented the development of diarrhea. Four of five mice showed no stool or brown formed stool, and only one mouse showed brown soft stool, while water treatment caused watery diarrhea in all five mice. The mean titer of antibody to RV in mice which received NMSO3 at 10 μg three times per day for 4 days was significantly lower than that of untreated, infected mice. NMSO3 is a promising candidate for the prophylactic treatment of human RVs.
Rotavirus, a member of the Reoviridae, is a nonenveloped virus which has a segmented, double-stranded RNA genome surrounded by three concentric protein layers. Human rotaviruses (HRV) are the major etiologic agents of severe dehydrating gastroenteritis in infants and young children worldwide (9). They cause more than 850,000 deaths per year in developing countries (6). To prevent the rotavirus infection, several attenuated live vaccines have been developed and subjected to extensive clinical trials. Among them, an oral tetravalent rhesus-human reassortant rotavirus vaccine was first licensed by the Food and Drug Administration. However, it was withdrawn from the market because of the rare but severe complication of intussusception (16).
For the treatment of rotavirus gastroenteritis, intravenous fluid supply has been used successfully for treatment of dehydration from diarrhea. However, in the case of severe inpatients and immunocompromised hosts who are suffering from prolonged diarrhea and fever, virus-specific treatment will be expected, if possible. Biomaterials, several compounds, and plant extracts have been found to be effective for suppression of rotavirus infection in vitro (3, 4, 5, 10, 12) and in vivo (17). Some of them have prevented HRV-induced diarrhea in suckling mice (4, 5) and in humans (14, 15), but none of them has yet been in clinical use.
NMSO3 is a sulfated sialyl lipid which is chemically synthesized. We have found that NMSO3 had inhibitory activity against HRV growth. In this report we describe (i) in vitro anti-HRV activity of NMSO3, (ii) inhibitory mechanism of NMSO3 against HRV, and (iii) protective efficacy of NMSO3 against HRV-induced diarrhea in a mouse model. (Part of the information in this report has been presented at the 48th Annual Meeting for the Japanese Society for Virologists [13 October, 2000, Mie, Japan]).
MATERIALS AND METHODS
Cells and viruses.
MA104 cells (African rhesus monkey kidney cells) were cultured in Eagle's minimal essential medium supplemented with 10% fetal calf serum. HRV strains Wa (serotype G1), S2, DS-1 (G2), MO (G3), and Hochi (G4) were propagated as described previously (19), and the virus titer was determined by a plaque assay. For experiments with the mouse diarrhea model, the MO strain was used and purified as previously described (2) with modifications. In brief, after two freeze-thaw cycles, MO strain-infected cells were centrifuged at 3,000 × g for 30 min and the supernatant was ultracentrifuged at 100,000 ×g for 3 h. The pellet was suspended in phosphate-buffered saline (PBS) containing a 1 mM concentration each of CaCl2 and MgCl2 and stored in aliquots at −80°C until use.
Chemicals.
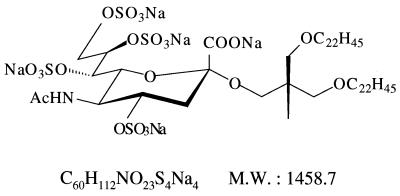
NMSO3, sodium [2,2-bis(docosyl-oxymethyl)propyl-5-acetoamido-3,5-dideoxyl-4,7,8,9-tetra-O-(sodium-oxy sulfonyl)-d-glycero-α-d-galacto-2-nonulopyranosid]onate (molecular weight, 1458.7), is a sulfated sialyl lipid, and its structural formula is shown in Fig. 1.NMSO3 was chemically synthesized and purified to more than 98% purity at the Central Research Institute of Nissin Food Products Co. Ltd. (Kusatsu, Shiga, Japan).
FIG. 1.
Chemical structure of NMSO3, sodium [2,2-bis(docosyl-oxymethyl)propyl-5-acetoamido-3,5-dideoxyl-4,7,8,9-tetra-O-(sodium-oxy sulfonyl)-d-glycero-d-galacto-2-nonulopyranosid]onate. M. W.,molecular weight.
Antiviral assays.
The inhibitory effect of NMSO3 on the replication of rotavirus was determined by the inhibition of virus-induced cytotoxicity in MA104 cells. In brief, a confluent monolayer culture of MA104 cells was infected with the Wa strain of rotavirus (100 PFU/well) with various concentrations of NMSO3 in a 96-well microplate and cultured for 3 days. The number of viable cells was indirectly determined colorimetrically by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as described previously (19). The 50% effective concentration (EC50), 50% cytotoxic concentration (CC50), and selectivity index (SI) were defined as previously described (19).
FFU assay.
The titer of virus of the Wa strain was also determined by an FFU assay. MA104 cells in a 96-well plate were infected with 10-fold serial dilutions of trypsin-treated virus stock and cultured for 24 h. The cells were fixed with methanol, washed with PBS, and reacted with 10,000-fold dilutions of rabbit anti-rotavirus (KU strain) serum for 30 min at room temperature. After washing, the cells were reacted with goat anti-rabbit immunoglobulin (IgG) antibody conjugated with horseradish peroxidase. Rotavirus-infected cells were visualized by diaminobenzidine, and the number of infected cells (=focus) was determined by microscopy to determine the FFU titer of the virus stock.
Time of addition experiments.
MA104 cells in a 96-well plate were pretreated with various concentrations of NMSO3 before virus adsorption (100 FFU/well) for 1 h or treated with NMSO3 during or after virus adsorption. The cells were cultured for 24 h, and virus-infected cells were visualized and counted as described above. The EC50 was defined as the concentration of NMSO3 required for reducing the number of focuses by 50%.
Plaque reduction assay.
The inhibitory effect of NMSO3 was also examined by plaque reduction assay in six-well plates as previously described (19). NMSO3 was added in the culture during the time of adsorption. The EC50 was defined as the concentration of NMSO3 required for reducing the number of plaques by 50%.
Virus binding assay.
Virus binding assay was performed with radio labeled purified Wa strain. Wa strain-infected MA104 cells were radiolabeled with 100 μCi of 35S-methionine for 12 h until a massive cytopathic effect was observed. The radiolabeled virus was purified with CsCl gradient by ultracentrifugation as described previously (2). Fractions of 100-μl aliquots were tested for virus titer by an FFU assay and for radioactivity. For a virus binding assay, a confluent monolayer of MA104 cells in a 96-well microplate was incubated with the 48 FFU of radiolabeled virus (2 × 104 cpm) in the presence or absence of NMSO3 for 1 h on ice, after which cells were washed free of medium and excess labeled virus. The cells were lysed with lysis solution (1% Triton-X, 0.15 M NaCl, 10 mM Tris-HCl), and bound virus was counted in a liquid scintillation counter (Aloka, Tokyo Japan). The percentage of bound viruses was calculated as (cpm. of membrane-bound virus/cpm. of total input Wa strain in medium) × 100.
Animals.
ICR suckling mice were purchased from Clea Japan, Inc. (Tokyo, Japan) and maintained in a laminar flow hood in the Animal Experiment Facility of the Fukushima Medical University. Animal experimentation guidelines of the Fukushima Medical University were followed.
Mouse diarrhea model.
We used a previously developed mouse model of rotavirus gastroenteritis to study the effect of NMSO3 on the development of diarrhea (4). Litters of 7-day-old mice were orally inoculated (50 μl) with 3 × 106 PFU of the MO strain by a gavage. Stools were examined daily for characteristics of diarrhea by gentle abdominal palpation beginning at one day after inoculation for 5 days. A six-point rating system was used to characterize diarrhea: 1, no stool; 2, normal brown formed stool; 3, soft brown stool; 4, soft-mucous brown-yellow stool; 5, muddy-mucous yellow stool; 6, liquid-mucous yellow stool. To determine the effective amount of NMSO3 for prevention of rotavirus-induced diarrhea, NMSO3 was given orally by a gavage (50 μl) three times per day (at 9 a.m., 3 p.m., and 9 p.m.) for 4 days starting 30 min before virus inoculation. To evaluate the initiation time of treatment, treatment with NMSO3 started 6, 9, 12, or 18 h after virus inoculation. In both experiments stools were examined and scored daily as described above, and the efficacy of NMSO3 was evaluated based on the prevention of development of diarrhea. Body weight of mice was measured every morning during experiments.
Immunofluorescence.
Mouse titers of antibody to the MO strain of HRV were determined by immunofluorescence. Mice were anesthesized, and blood was collected from the axillary artery 14 days after HRV inoculation. MA104 cells were cultured on 24-well multispotted glass slides and infected with MO strain at a multiplicity of infection of 0.5 for 24 h. Slides were washed with PBS, dried, and fixed with cold acetone. The infected cells were reacted with twofold serial dilutions of the serum for 30 min at room temperature. After two washes with PBS, cells were incubated with fluorescent isothiocyanate-conjugated rabbit anti-mouse IgG antibody (1:200; DAKO JAPAN, Kyoto, Japan). After washing, the titer of antibody was determined by fluorescence microscopy. The antibody titer was expressed as the highest serum dilution showing a detectable signal.
Statistical analysis.
Statistical analyses have been performed by SPSS (SPSS Inc., Chicago Ill.). Analysis of variance was used to examine the difference in serum IgG titer to HRV among infected mice receiving various doses of NMSO3.
RESULTS
Inhibitory effect of NMSO3 on the HRV replication.
Inhibitory effect of NMSO3 on the HRV (Wa strain) replication was examined by MTT assays. NMSO3 showed highly inhibitory activity against HRV with an EC50 of 0.82 μg/ml, moderately low cytotoxicity with a CC50 of 153 μg/ml, and an acceptable SI of 186. For the strategy to be of value in a clinical application, it was determined whether exposure of NMSO3 to HCl (pH 2) may cause loss of activity or not. The inhibitory activity of NMSO3 was not reduced on prior exposure of NMSO3 to HCl for 30 min at pH 2 (EC50, 0.82 μg/ml; CC50, 153 μg/ml; SI, 186).
Inhibitory effect of NMSO3 on virus adsorption.
To explore the inhibitory mechanism of NMSO3 to HRV (Wa strain), time-of-addition experiments were performed by FFU assays in which only one cycle of virus replication occurs. Various concentrations of NMSO3 were added to MA104 cells before (pretreatment), during, or after virus adsorption when it is assumed the nonadsorbed virus has been removed, and the inhibitory effects were evaluated by EC50. As shown in Table 1, NMSO3 significantly inhibited the virus replication at the EC50 of 5.2 μg/ml when added during virus adsorption compared with addition before or after virus adsorption (P < 0.01 or P < 0.05, respectively [t test]). Treatment of MA104 cells with NMSO3 after virus adsorption also significantly inhibited the virus growth at an EC50 of 14 μg/ml compared with the case of pretreatment (EC50, 57 μg/ml; P < 0.01 [t test]).
TABLE 1.
Time-of-addition experiment with NMSO3 in FFU assay
| Time of addition of NMSO3 (h) | EC50 (μg/ml)a | P value |
|---|---|---|
| −1-0e | 57 ± 6.0 | <0.01b |
| 0-1.5 | 5.2 ± 0.55 | <0.05c |
| 1.5-24 | 14 ± 3.6 | <0.01d |
EC50s were determined by three independent experiments.
Compared with the case treated during 0 to 1.5 h.
Compared with the case treated during 1.5 to 24 h.
Compared with the case treated during −1 to 0 h.
0 h, time of rotavirus inoculation.
Inhibitory effect of NMSO3 on growth of four serotypes of HRV.
Since there are four major serotypes of HRV which cause gastroenteritis in humans worldwide, we further examined the inhibitory effect of NMSO3 on adsorption of the four serotypes of HRV by a plaque reduction assay. As shown in Table 2, NMSO3 also inhibited the adsorption of the other three serotypes of HRV as actively as with the Wa strain, with EC50s ranging from 1.7 to 4.7 μg/ml. On the other hand, NMSO3 had no effect on a simian rotavirus, SA11.
TABLE 2.
Effect of NMSO3 on virus adsorption of four serotypes of HRV determined by plaque assay
| Serotype | Strain | EC50 (μg/ml)a |
|---|---|---|
| 1 | Wa | 2.1 ± 0.5 |
| 2 | DS-1 | 4.7 ± 0.7 |
| S-2 | 1.7 ± 0.4 | |
| 3 | MO | 2.2 ± 0.7 |
| 4 | Hosokawa | 1.7 ± 0.5 |
| 3 | SA11 (simian RV) | >100 |
Mean values were determined for three independent experiments.
Effect of NMSO3 on the binding of the Wa strain to MA104 cells.
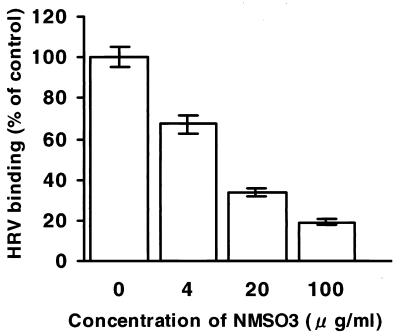
Since NMSO3 inhibited the virus adsorption step, we further examined whether NMSO3 inhibits the binding of the radiolabeled purified Wa strain to MA104 cells, and the result is shown in Fig. 2.NMSO3 blocked the binding of the Wa strain to the cells in a dose-dependent manner, and 66% inhibition of control binding occurred at an NMSO3 concentration of 20 μg/ml.
FIG. 2.
Effect of NMSO3 on binding of the Wa strain to MA104 cells. Triplicate cultures of MA104 cells were incubated with 35S-labeled Wa strain in various concentrations of NMSO3. Data represent mean percentages (± standard deviations) of membrane-bound virus.
Protective efficacy of NMSO3 against rotavirus-induced diarrhea in a mouse model.
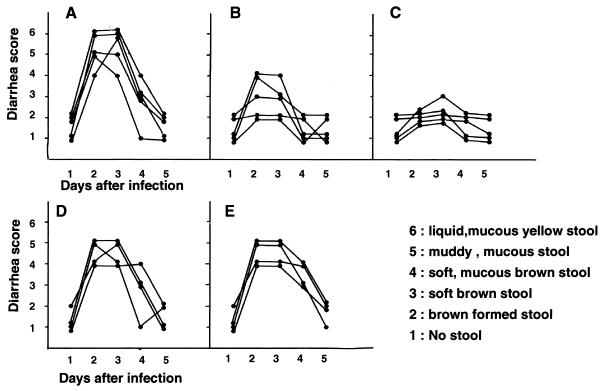
Initially groups of four to five pups were orally inoculated with 3 × 106 PFU of the MO strain. Water treatment caused diarrhea (diarrhea index [DI] of 4, 5, or 6) 2 to 3 days after inoculation (Fig. 3Aand 4A).Signs of diarrhea were not seen in all negative controls, which included sham inoculations with a mock viral purification (data not shown). To determine the effective amount of NMSO3 for prevention of rotavirus-induced diarrhea, mice were orally given 50, 10, 2, or 0.4 μg of NMSO3 three times per day for 4 days. All mice given 10 μg of NMSO3 did not develop diarrhea (Fig. 4B). Only one of five mice showed a soft brown stool, and the others showed no stool or a brown formed stool (Fig. 3C) during the experimental course, indicating that diarrhea in these mice was prevented. Mice given 50 μg of NMSO3 showed partial protection (Fig. 3B). Two of five mice developed soft mucous diarrhea (DI of 4), but the others showed brown formed to brown soft stool during the course. On the contrary, 2 or 0.4 μg of NMSO3 had little effect on the development of diarrhea (Fig. 3D and E). All mice developed soft-mucous to muddy-mucous diarrhea (DI, 4 or 5) but not liquid mucous diarrhea (DI, 6). All mice recovered at the fifth day after inoculation without mortality. There was no significant difference in the body weight of mice after infection in each group (data not shown).
FIG. 3.
Protective effect of NMSO3 on HRV-induced diarrhea in suckling mice. Litters of 7-day-old mice were given orally either water (A) or 50 (B), 10 (C), 2 (D), or 0.4 (E) μg of NMSO3 three times a day for 4 days starting 30 min before virus inoculation with 3 × 106 PFU (MO strain)/mouse. A six-point rating system was used to characterize diarrhea: 1, no stool; 2, brown formed stool; 3, soft brown stool; 4, soft-mucous brown-yellow stool; 5, muddy-mucous yellow stool; 6, liquid yellow stool.
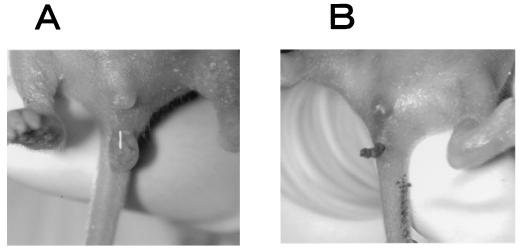
FIG. 4.
Effect of NMSO3 on HRV-induced diarrhea in suckling mice. Seven-day-old mice were given orally either water (A) or 10 μg of NMSO3 (B) three times a day for 4 days starting 30 min before virus inoculation with 3 × 106 PFU (MO strain)/mouse. Stool was inspected 2 days after virus inoculation.
To further determine the effective initiation time of treatment with NMSO3 after virus inoculation, mice were treated with 10 μg of NMSO3 starting 30 min before infection and 6, 12, or 18 h after virus inoculation until the fourth day of infection. Treatment starting 6, 12, or 18 h after virus inoculation did not prevent the mice from developing diarrhea. All mice of these groups developed diarrhea with a DI of over 4. All mice treated with NMSO3 starting 30 min before infection were protected from diarrhea, as is seen in Fig. 3C.
Effect of NMSO3 on serum IgG responses of mice to the HRV MO strain in a mouse diarrhea model.
Serum titers of IgG specific to the MO strain 14 days after virus inoculation were determined by immunofluorescence to estimate the extent of suppression of viral replication in mice treated with NMSO3 starting 30 min before virus inoculation. The resulting antibody titers are shown in Table 3. The mean titer of IgG for the MO strain in the infected mice without NMSO3 treatment was significantly higher than those in mice treated with NMSO3, 10 and 50 μg/dose, three times per day (P < 0.05; [t test]) but not significantly different from a group of mice treated with 2 μg/dose.
TABLE 3.
Effect of NMSO3 administration on serum IgG responses to HRV in mice inoculated with the MO strain.
| Dose of NMSO3 (×3/day, 4 days) (μg) | na | Serum IgG geometric mean titerb (0.1) (2mean ± SD) |
|---|---|---|
| None | 7 | 24.3 ± 1.8 |
| 2 | 5 | 23.8 ± 1.6 |
| 10 | 7 | 21.2 ± 1.6 c |
| 50 | 5 | 20.8 ± 1.3 c |
n, no. of mice.
Antibody titers were determined by immunofluorescence.
P < 0.05 compared with results for untreated group.
DISCUSSION
In the present study, we have demonstrated that NMSO3, a sulfated sialyl lipid, has a potent inhibitory activity against four serotypes (G1 to G4) of HRV in vitro with a low EC50 of 1.5 to 4.7 μg/ml (1.0 to 3.0 μM) and an acceptable SI of 186. These four serotypes are the major causes of HRV gastroenteritis (1, 18, 20). These EC50s are the lowest values for drugs which have been reported to be inhibitory to HRV. Time-of-addition experiments whose results are shown in Table 1 suggested that the primary inhibitory mechanism of NMSO3 is adsorption inhibition. In addition, experiments on virus binding showed that NMSO3 blocked the binding of virus to MA104 cells in concentrations which are consistent with the results of a plaque assay. Furthermore, pretreatment of MA104 cells with NMSO3 in the time-of-addition experiment showed little inhibitory effect on virus growth. Therefore, taken together, these findings suggest that NMSO3 inhibits virus attachment to the cells by binding to VP4 and/or VP7 molecules of the outer layer of rotavirus, although a possibility of inhibition of virus attachment by binding of NMSO3 to cell receptor(s) cannot completely be excluded. As shown in Table 1, NMSO3 also inhibited focus formation when added after virus adsorption compared with results when added before virus adsorption. This inhibitory effect may be due to the binding of NMSO3 to the viruses which have bound on cell membranes but not yet been incorporated into the cells, followed by the inhibition of entry of the viruses. To explore which viral protein NMSO3 would bind to, we have tested whether NMSO3 could inhibit a binding of some VP4- or VP7-specific monoclonal antibodies to Wa-infected MA104 cells. However, the binding of none of the VP4- or VP7-specific monoclonal antibodies in our hands was inhibited by NMSO3 (data not shown).
The mechanism of binding of NMSO3 to VP4 and/or VP7 is unknown. NMSO3 may bind to the specific sites of VP4 and/or VP7 of HRVs by either hydrophobic association of lipid chains or negative charge of sulfate residues of NMSO3 followed by an interference with the binding of VP4 and/or VP7 to cellular receptor(s) by steric hindrance. On the contrary, NMSO3 did not inhibit growth of the SA11 strain. The reason is unknown. NMSO3 may not bind to VP4 and/or VP7 of SA11, since there are approximately 28.7 and 25.2% differences between SA11 and HRV G3 in amino acid sequences of VP4 and VP7, respectively (sequence data obtained from GenBank). Another interpretation is that NMSO3 may bind to a site other than the receptor binding site of VP4 and/or VP7 of both viruses, and the NMSO3-bound viral protein(s) of the MO strain but not of SA11 could not bind to the receptor, probably because of the conformational change.
We have recently defined that NMSO3 also has potent inhibitory activities against respiratory syncytial virus, with an EC50 of 0.12 to 0.32 μM (plaque reduction assay) (11), and adenoviruses, with an EC50 of approximately 1 μM (MTT assay) (8). On the other hand, NMSO3 did not inhibit growth of influenza virus A, parainfluenza virus 2, coxsackie B3 virus, cytomegalovirus, or herpes simplex virus 1 (EC50, >100 μM; Takahashi and Terada, unpublished data). Since NMSO3 inhibited the adsorption and penetration step of RSV and adenovirus, it was suggested that NMSO3 would bind F and/or G proteins of RSV and the fiber and/or capsomer of adenovirus. It is of interest to define whether these viral outer-layer proteins commonly have characteristics similar to those of the structure to which NMSO3 may bind.
In the present HRV-induced diarrhea model in mice, prophylactic oral administration of NMSO3 to suckling mice starting 30 min before inoculation of the MO strain prevented the development of diarrhea. This result suggests that NMSO3 would not be inactivated in the gastrointestinal tract and would prevent the virus from attaching to intestinal mucosal cells by binding to the virus. Administration of NMSO3 at a dosage of even 50 μg three times per day proved less effective, with mild diarrhea developing in two of five mice, than at 10 μg three times per day. The reason for this decreased effectiveness is unknown. Further mouse studies on a large scale will be necessary to answer this question. Administration of NMSO3 after virus inoculation did not prevent mice from developing diarrhea. This finding suggests that in this mouse diarrhea model it would be essential to block the initial infection in the intestine to prevent diarrhea. Namely, completion of the initial infection would be sufficient for the development of diarrhea.
To investigate whether NMSO3 really inhibits viral growth in the murine gastrointestinal tract, we initially examined the titer of virus in the whole intestines of mice at short time intervals after infection with 3 × 106 PFU of the MO strain as described by Gouvea et al. (7). However, we could not obtain any infectious virus from the intestine by plaque assay. Therefore, we measured levels of IgG to whole virus in serum by an immunofluorescence assay because low levels of viral growth may reflect a low-level antibody response. The mean titer of antibody for mice which received NMSO3 at a dosage of 10 or 50 μg, three times per day for 4 days, was significantly lower than that for untreated, infected mice (Table 3), suggesting that NMSO3 inhibited viral replication in the intestines of the treated mice, which was followed by low-level antibody responses.
Among anti-HRV agents previously investigated in vitro and in vivo, several agents prevented the development of HRV-induced diarrhea in mouse models or reduced HRV-induced diarrhea in humans (14, 15). These agents include egg yolk anti-HRV immunoglobulins (4), Polysaccharide-Kureha, a protein-bound polysaccharide prepared from the extract of the mycelia of Coriolus versicolor of Basidiomycetes (4), a cysteine protease inhibitor, ovocystatin, and a serine protease inhibitor, aprotinin (5). However, none of them has yet been approved for clinical use. To our knowledge, NMSO3 is the first chemically synthesized adsorption inhibitor which could prevent HRV-induced diarrhea in mice.
In the present mouse diarrhea model, prophylactic administration of NMSO3 prevented the development of diarrhea. Therefore, prophylactic use of NMSO3 in cases of epidemics in a nursery would be expected if NMSO3 shows no toxicity for humans. In considering clinical application in HRV gastroenteritis, therapeutic treatment should be necessary. Since in infantile HRV gastroenteritis HRVs continue to be excreted into the stool for 4 to 5 days after onset (13), there should be several cycles of viral replication in the intestine for 4 to 5 days. Therefore, in humans it would be possible for NMSO3 to inhibit viral replication in the intestine, and it could shorten the duration of fever and diarrhea. NMSO3 would be a promising candidate for the treatment of HRV infections in humans.
Acknowledgments
We are grateful to A. Imura for excellent technical assistance, M. Miura for preparation of the manuscript, and K. Christensen for reviewing the manuscript.
REFERENCES
- 1.Chakravarti, A., S. Kumar, S. K. Mittal, and S. Broor. 1992. Clinical and epidemiological features of acute gastroenteritis caused by human rotavirus subgroups. J. Diarrhoeal Dis. Res. 10:21-24. [PubMed] [Google Scholar]
- 2.Chen, D., and R. F. Ramig. 1992. Determinants of rotavirus stability and density during CsCl purification. Virology 186:228-237. [DOI] [PubMed] [Google Scholar]
- 3.Clark, K. J., P. G. Grant, A. B. Sarr, J. R. Belakere, C. L. Swaggerty, T. D. Phillips, and G. N. Woode. 1998. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet. Microbiol. 63:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebina, T., K. Tsukada, K. Umezu, M. Rose, K. Tsuda, H. Hatta, M. Kim, and T. Yamamoto. 1990. Gastroenteritis in suckling mice caused by human rotavirus can be prevented with egg yolk immunoglobulin (IgY) and treated with a protein-bound polysaccharide preparation (PSK). Microbiol. Immunol. 34:617-629. [DOI] [PubMed]
- 5.Ebina, T., and K. Tsukada. 1991. Protease inhibitors prevent the development of human rotavirus-induced diarrhea in suckling mice. Microbiol. Immunol. 35:583-588. [DOI] [PubMed] [Google Scholar]
- 6.Glass, R. I., J. Gentsch, and J. C. Smith. 1994. Rotavirus vaccines: success by reassortment? Science 265:1389-1391. [DOI] [PubMed] [Google Scholar]
- 7.Gouvea, V. S., A. A. Alencar, O. M. Barth, L. de Dastro, A. M. Fialho, H. P. Araujo, Majerowicz, and H. G. Pereira. 1986. Diarrhoea in mice infected with a human rotavirus. J. Gen. Virol. 67:577-581. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko, H., K. Kato, S. Mori, and S. Shigeta. 2001. Antiviral activity of NMSO3 against adenovirus in vitro. Antivir. Res. 52:281-288. [DOI] [PubMed]
- 9.Kapikian, A. Z. 1996. Overview of viral gastroenteritis. Arch. Virol. 12(Suppl.):7-19. [DOI] [PubMed] [Google Scholar]
- 10.Kiefel, M. J., B. Beisner, S. Bennett, I. D. Holmes, and M. Itzstein. 1996. Synthesis and biological evaluation of N-acetylneuraminic acid-based rotavirus inhibitors. J. Med. Chem. 39:1314-1320. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, K., S. Mori, K. Tomita, K. Ohno, K. Takahashi, S. Shigeta, and M. Terada. 2000. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antivir. Res. 47:41-51. [DOI] [PubMed] [Google Scholar]
- 12.Koketsu, M., T. Nitoda, H. Sugino, L. R. Juneja, M. Kim, T. Yamamoto, N. Abe, T. Kajimoto, and C. H. Wong. 1997. Synthesis of a novel sialic acid derivative (sialylphospholipid) as an antirotaviral agent. J. Med. Chem. 40:3332-3335. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs, A., L. Chan, C. Hotrakitya, G. Overturf, and B. Portnoy. 1987. Rotavirus gastroenteritis. Clinical and laboratory features and use of the Rotazyme test. Am. J. Dis. Child 141:161-166. [DOI] [PubMed] [Google Scholar]
- 14.Losonsky, G. A., J. P. Johnson, J. A. Winkelstein, and R. H. Yolken. 1985. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis. A pharmacokinetic and functional analysis. J. Clin. Investig. 76:2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra, A. K., D. Mahalanabis, H. Ashraf, L. Unicomb, R. Eeckels, and S. Tzipori. 1995. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double-blind, controlled clinical trial. Acta Paediatr. 84:996-1001. [DOI] [PubMed] [Google Scholar]
- 16.Nakagomi, T. 2000. Rotavirus infection and intussusception: a view from retrospect. Microbiol. Immunol. 44:619-628. [DOI] [PubMed] [Google Scholar]
- 17.Newburg, D. S., J. A. Peterson, G. M. Ruiz-Palacios, D. O. Matson, A. L. Morrow, J. Shults, M. L. Guerrero, P. Chaturvedi, S. O. Newburg, C. D. Scallan, M. R. Taylor, R. L. Ceriani, and L. K. Pickering. 1998. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 351:1815-1816. [DOI] [PubMed] [Google Scholar]
- 18.Steele, A. D., P. Bos, and J. J. Alexander. 1988. Clinical features of acute infantile gastroenteritis associated with human rotavirus subgroup I and II. J. Clin. Microbiol. 26:2647-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi, K., M. Matsuda, K. Ohashi, K. Taniguchi, O. Nakagomi, K. Okutani, N. Sato, Y. Abe, and S. Shigeta. 2001. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antivir. Res. 49:15-24. [DOI] [PubMed] [Google Scholar]
- 20.Uhnoo, I., and L. Svensson. 1986. Clinical and epidemiological features of acute infantile gastroenteritis associated with human rotavirus subgroups 1 and 2. J. Clin. Microbiol. 23:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]