Abstract
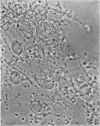
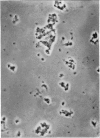
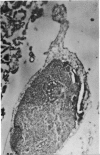
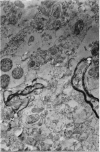
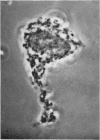
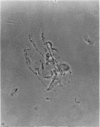
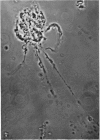
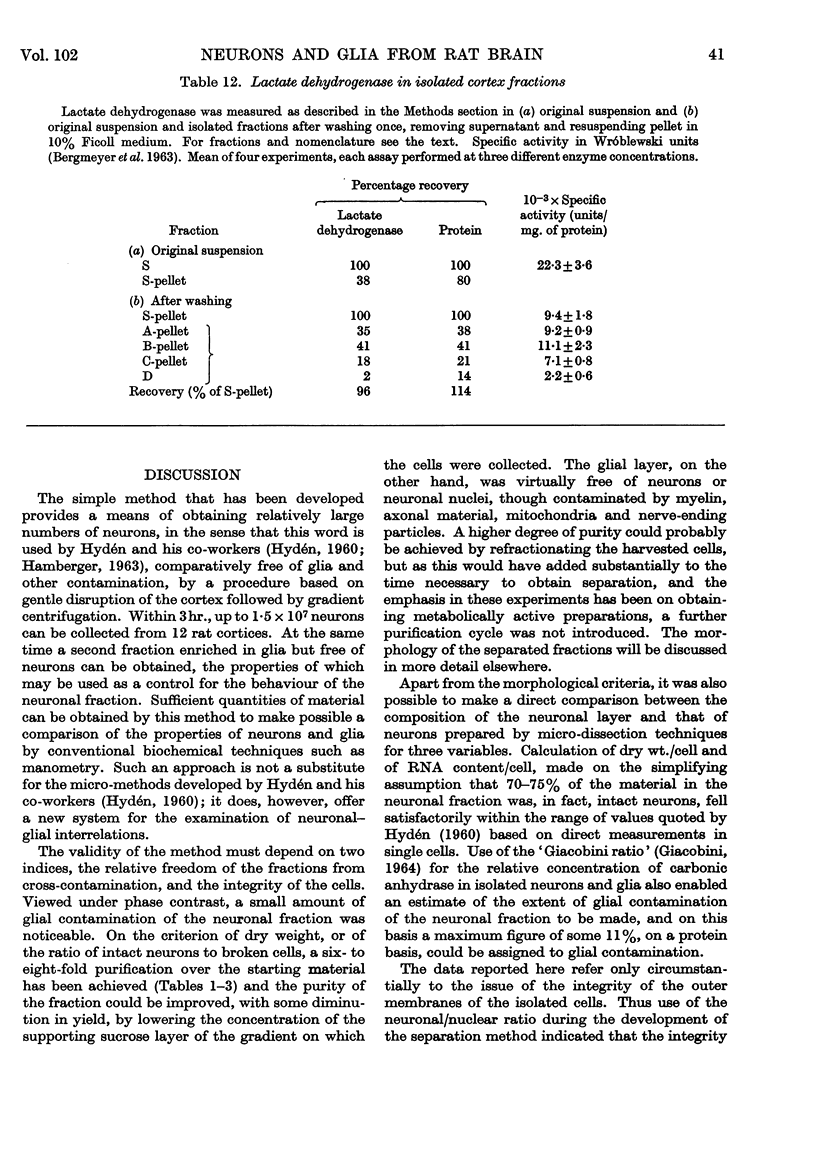
1. A procedure has been developed for the separation of intact metabolically active neuronal and glial cells in bulk from rat cerebral cortex. Separation depended on dispersion of the tissue in a Ficoll medium followed by centrifugation on a discontinuous Ficoll gradient. Up to 1·5×107 neuronal cells could be collected from 12 brains within 3hr. The morphological appearance of these cells seemed good, and the fraction was 8·5-fold purified in terms of dry weight. Average dry weight per neuron was 2300μμg. Maximum glial contamination of the neuronal fraction was 11% as determined by carbonic anhydrase measurements. The glial fraction was free from neurons but contained various subcellular contaminants. 2. Concentrations of nucleic acids, phospholipid, protein and phosphoprotein were determined in the separated fractions. The neuronal fraction was richer than the glial in all except phospholipid. Succinate dehydrogenase was equally distributed between neurons and glia but the neuronal fraction was 1·8-fold enriched in cytochrome oxidase. 3. Measurement of respiration by the cells showed an endogenous uptake of 117mμmoles of oxygen/mg./hr. in neurons, and 173mμmoles of oxygen/mg./hr. in glia. Addition of substrate at 10mm stimulated uptake to similar values in both fractions. With glucose it was 390, with pyruvate 355, and with glutamate 215mμmoles of oxygen/mg./hr. This represented a larger stimulation of neuronal than of glial respiration compared with the basal level. 4. Respiration in cell suspensions was 70–80% of that of slices, whereas fractionated tissue homogenates had respiratory rates of only one-third those of the cell suspensions. Lactate dehydrogenase content of cell suspensions was maintained during gradient centrifugation and washing. 5. The possible uses of isolated cell preparations are discussed.
Full text
PDF


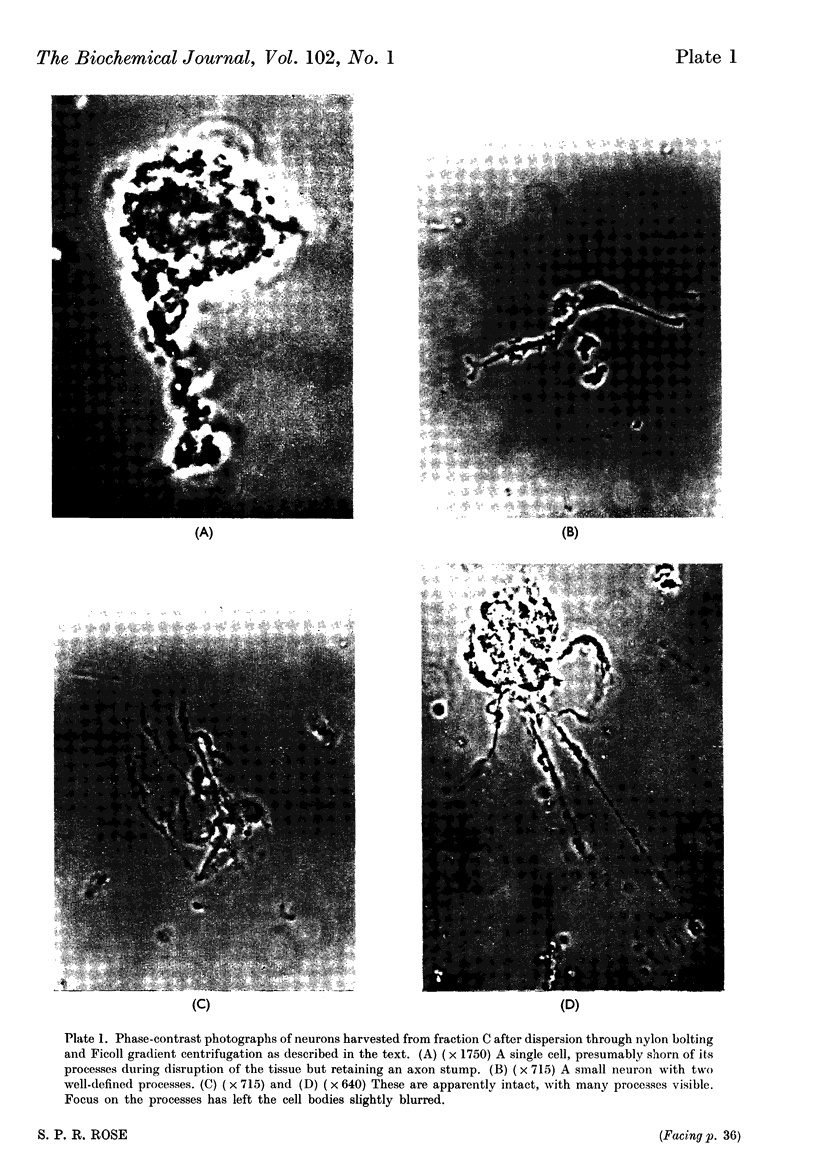
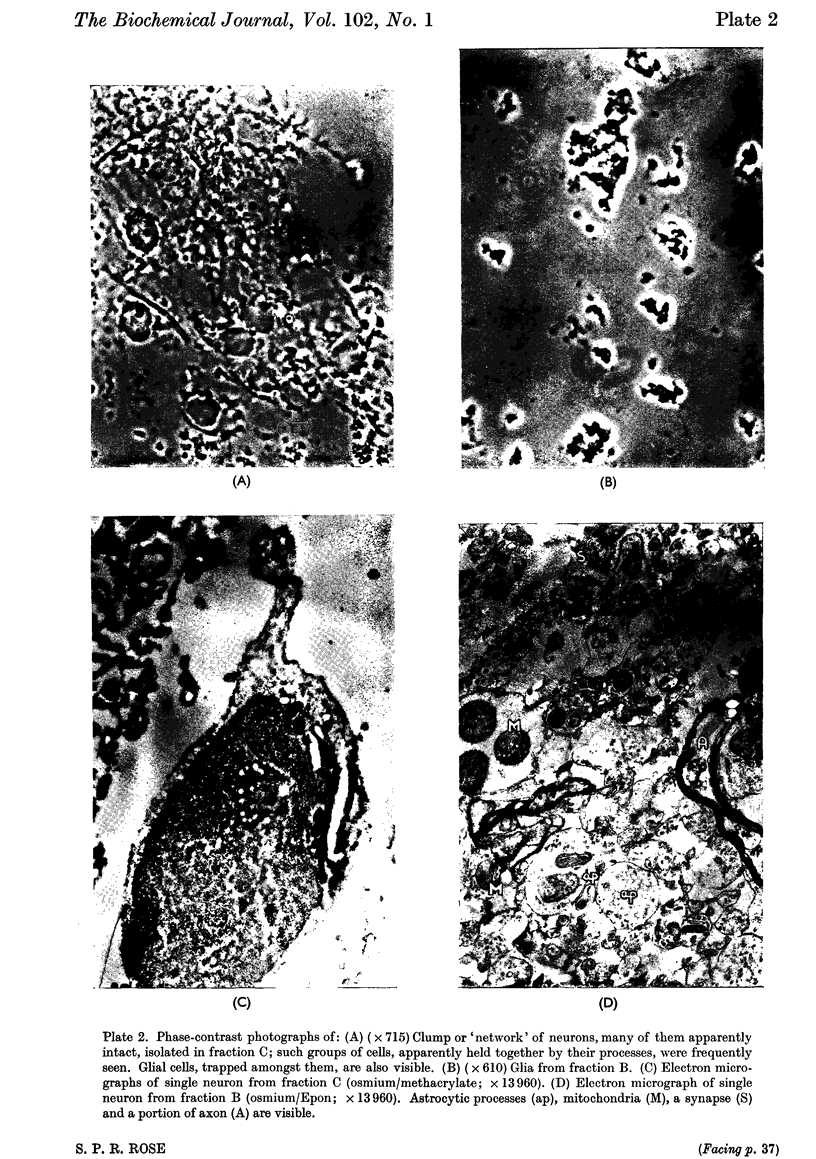

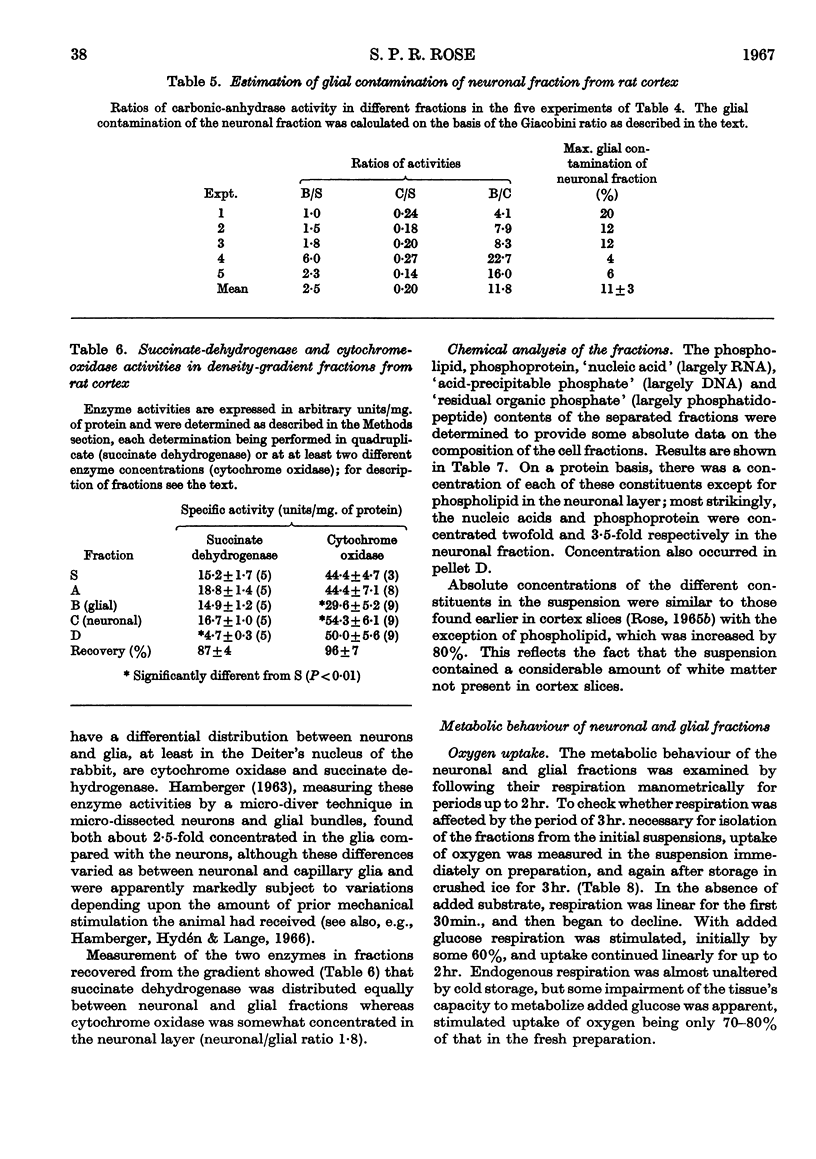
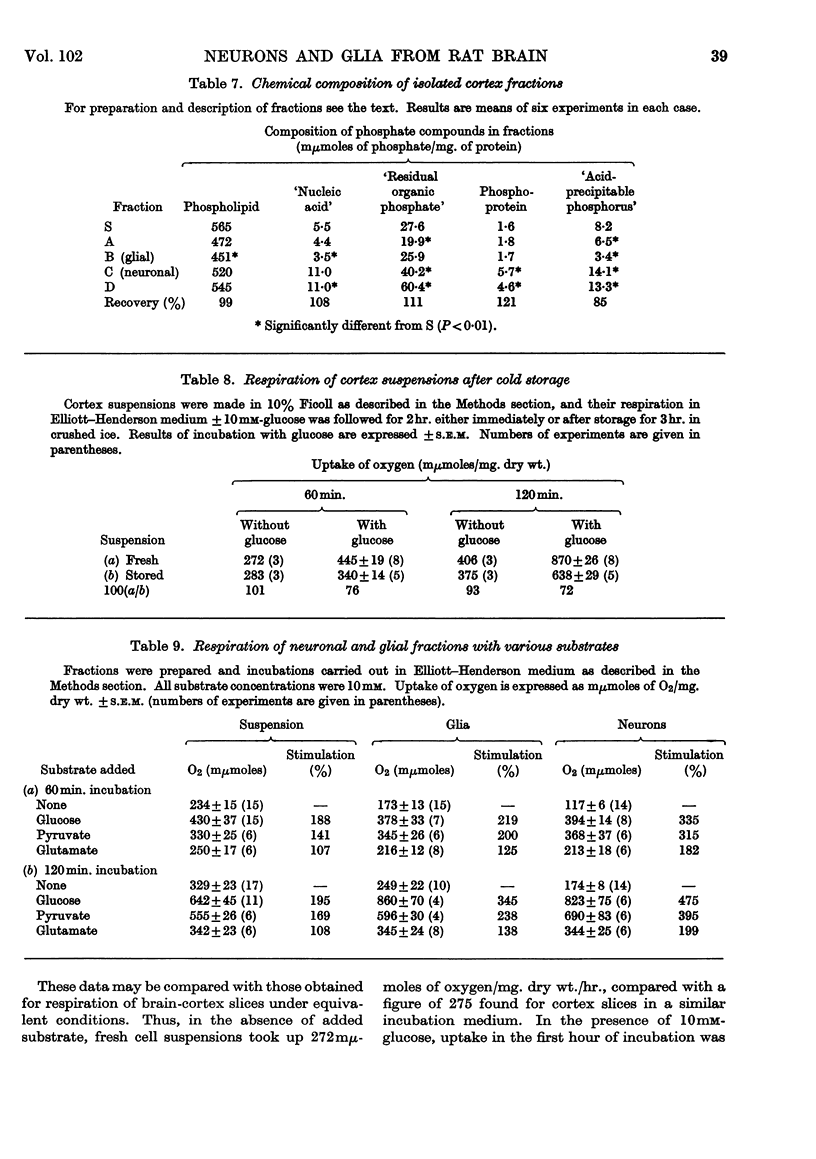



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTSSON P. A., BAIRD G. D. Counter-current distribution of cells. Exp Cell Res. 1962 Nov;28:296–322. doi: 10.1016/0014-4827(62)90285-9. [DOI] [PubMed] [Google Scholar]
- BRANSTER M. V., MORTON R. K. Isolation of intact liver cells. Nature. 1957 Dec 7;180(4597):1283–1284. doi: 10.1038/1801283a0. [DOI] [PubMed] [Google Scholar]
- CHU L. W. A cytological study of anterior horn cells isolated from human spinal cord. J Comp Neurol. 1954 Apr;100(2):381–413. doi: 10.1002/cne.901000206. [DOI] [PubMed] [Google Scholar]
- Hamberger A., Hydén H., Lange P. W. Enzyme changes in neurons and glia during barbiturate sleep. Science. 1966 Mar 18;151(3716):1394–1395. doi: 10.1126/science.151.3716.1394. [DOI] [PubMed] [Google Scholar]
- KOREY S. R., ORCHEN M., BROTZ M. Studies of white matter. I. Chemical constitution and respiration of neuroglial and myelin enriched fractions of white matter. J Neuropathol Exp Neurol. 1958 Jul;17(3):430–438. [PubMed] [Google Scholar]
- LINDSKOG S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta. 1960 Apr 8;39:218–226. doi: 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATEYKO G. M., KOPAC M. J. CYTOPHYSICAL STUDIES ON LIVING NORMAL AND NEOPLASTIC CELLS. Ann N Y Acad Sci. 1963 Aug 5;105:185–285. doi: 10.1111/j.1749-6632.1963.tb42979.x. [DOI] [PubMed] [Google Scholar]
- ROBINS E., SMITH D. E., EYDT K. M. The quantitative histochemistry of the cerebral cortex. I. Architectonic distribution of ten chemical constituents in the motor and visual cortices. J Neurochem. 1956 May;1(1):54–67. doi: 10.1111/j.1471-4159.1956.tb12054.x. [DOI] [PubMed] [Google Scholar]
- ROOTS B. I., JOHNSTON P. V. NEURONS OF OX BRAIN NUCLEI: THEIR ISOLATION AND APPEARANCE BY LIGHT AND ELECTRON MICROSCOPY. J Ultrastruct Res. 1964 Apr;10:350–361. doi: 10.1016/s0022-5320(64)80014-9. [DOI] [PubMed] [Google Scholar]
- ROSE S. P. EFFECTS OF OUABAIN, GLUTAMATE AND CATIONS ON PHOSPHATE INCORPORATION IN BRAIN SLICES. Biochem Pharmacol. 1965 Apr;14:589–601. doi: 10.1016/0006-2952(65)90231-5. [DOI] [PubMed] [Google Scholar]
- ROSE S. P. The localization of cerebral phosphoprotein phosphatase. Biochem J. 1962 Jun;83:614–622. doi: 10.1042/bj0830614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport C., Howze G. B. Dissociation of adult mouse liver by sodium tetraphenylboron, a potassium complexing agent. Proc Soc Exp Biol Med. 1966 Apr;121(4):1010–1016. doi: 10.3181/00379727-121-30951. [DOI] [PubMed] [Google Scholar]
- Rose S. P. Preparation of enriched fractions from cerebral cortex containing isolated, metabolically active neuronal cells. Nature. 1965 May 8;206(984):621–622. doi: 10.1038/206621a0. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWER D. B., ELLIOTT K. A. C. Activity of acetylcholine system in cerebral cortex of various unanesthetized mammals. Am J Physiol. 1952 Mar;168(3):747–759. doi: 10.1152/ajplegacy.1952.168.3.747. [DOI] [PubMed] [Google Scholar]