Abstract
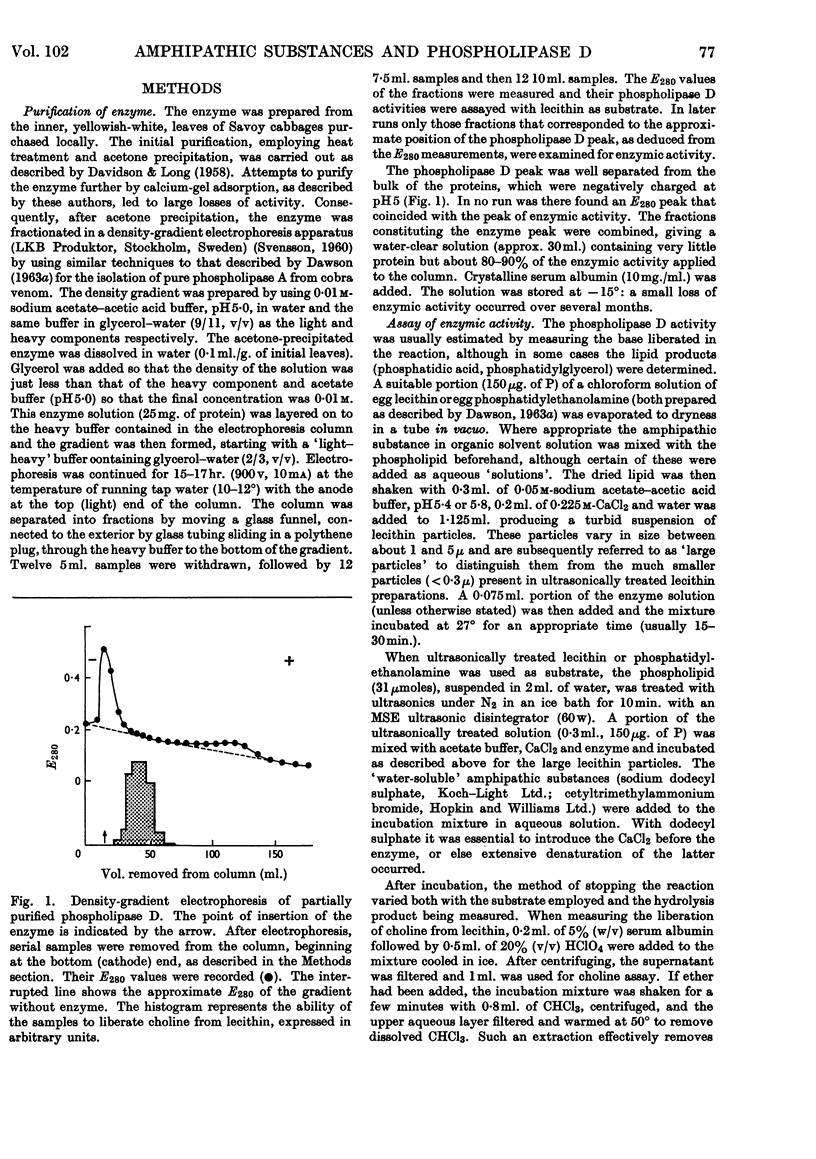
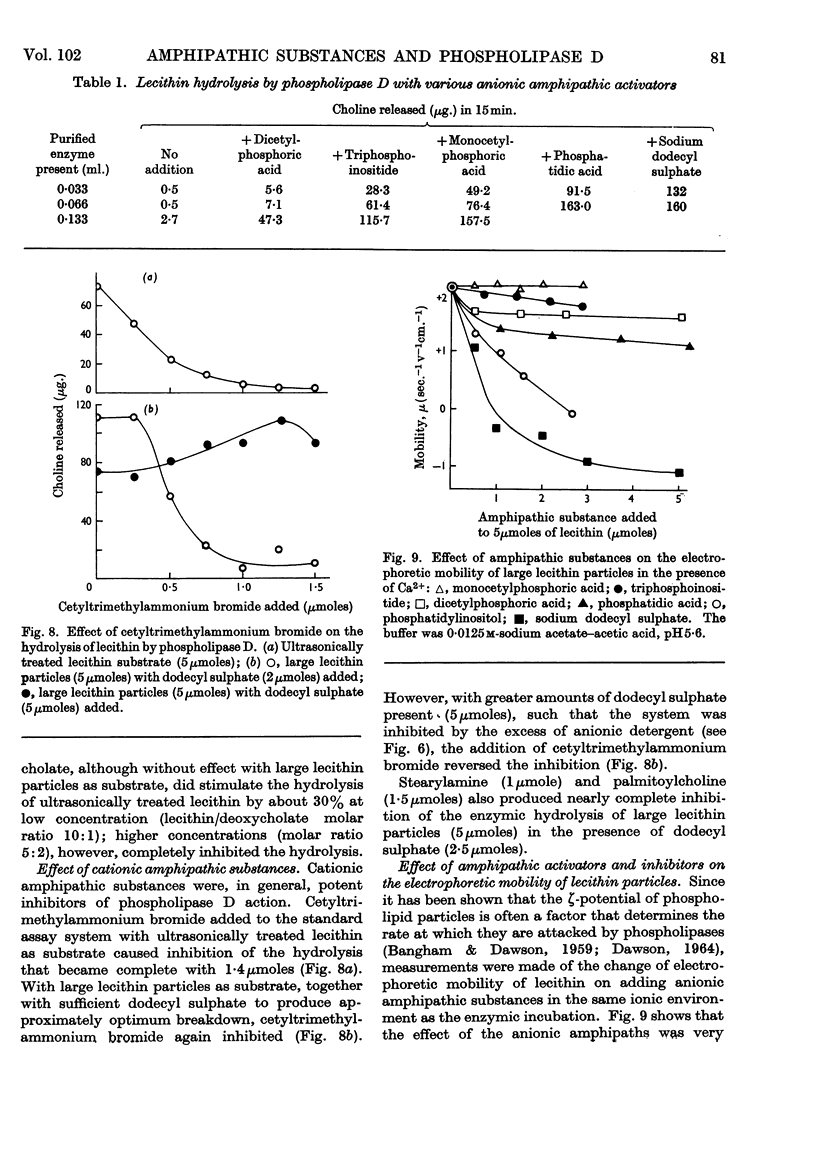
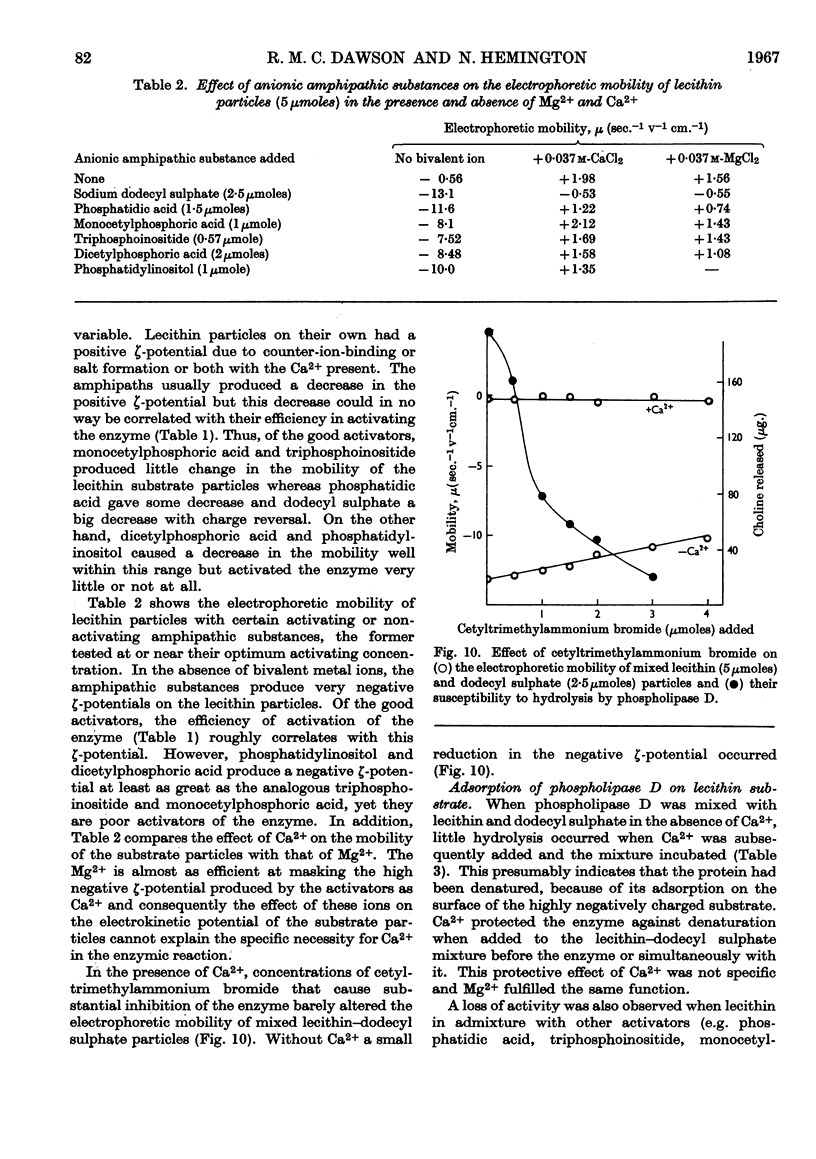
1. The soluble phospholipase D of cabbage was purified by heat treatment, acetone precipitation and electrophoresis on a density gradient of aqueous glycerol. 2. The purified enzyme slowly attacked a lecithin suspension whereas ultrasonically treated lecithin was hydrolysed more rapidly. 3. Diethyl ether stimulated the hydrolysis of both the lecithin suspension and ultrasonically treated lecithin. 4. Ca2+ was essential for the hydrolysis (optimum about 0·04m); it could not be replaced by Mg2+ or cationic amphipathic substances. 5. The reaction had a sharp pH optimum at pH5·4, irrespective of the physical form of the lecithin substrate or the activator used. 6. Anionic amphipathic substances such as dodecyl sulphate, phosphatidic acid, triphosphoinositide and monocetyl phosphoric acid, were potent activators of the reaction: other acidic lipids were relatively inactive. 7. Cationic amphipathic substances inhibited the hydrolysis; however, they also reversed the inhibition caused by using an excess of anionic amphipathic substance as activator. 8. The activation produced by amphipathic substances could not be correlated with their effect on the ζ-potential or size of the substrate particles. 9. The addition of activating anionic amphipaths to lecithin induces the latter to adsorb enzyme from solution. In the absence of Ca2+ the enzyme is denatured on the highly negatively charged surface, but in the presence of Ca2+ (or Mg2+) it is protected from denaturation. It is suggested that this adsorption is an essential prerequisite for ready enzymic hydrolysis. 10. The hydrolysis of lecithin by the enzyme was strongly inhibited by protamine sulphate (0·1mg./ml.) and by choline and ethanolamine. 11. Ultrasonically treated phosphatidylethanolamine, or mixed particles of phosphatidylethanolamine plus dodecyl sulphate, were slowly attacked by the enzyme provided that Ca2+ was present.
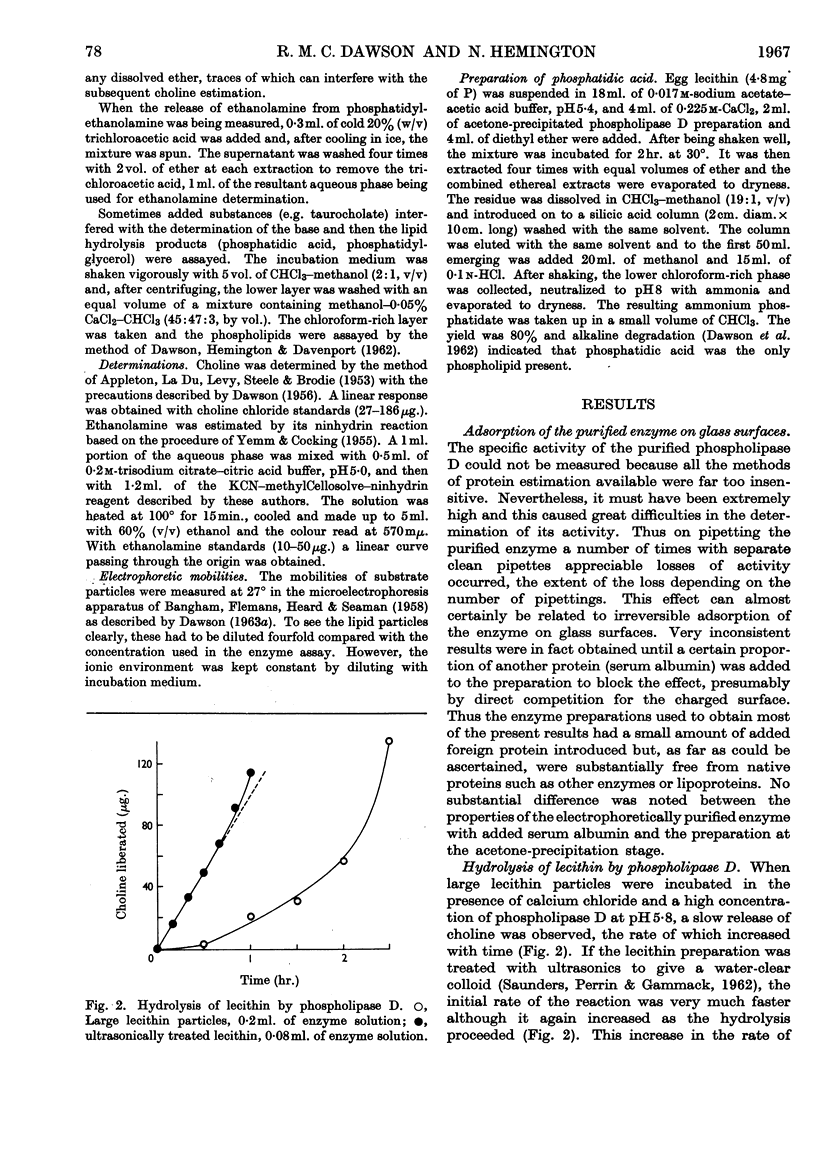
Full text
PDF
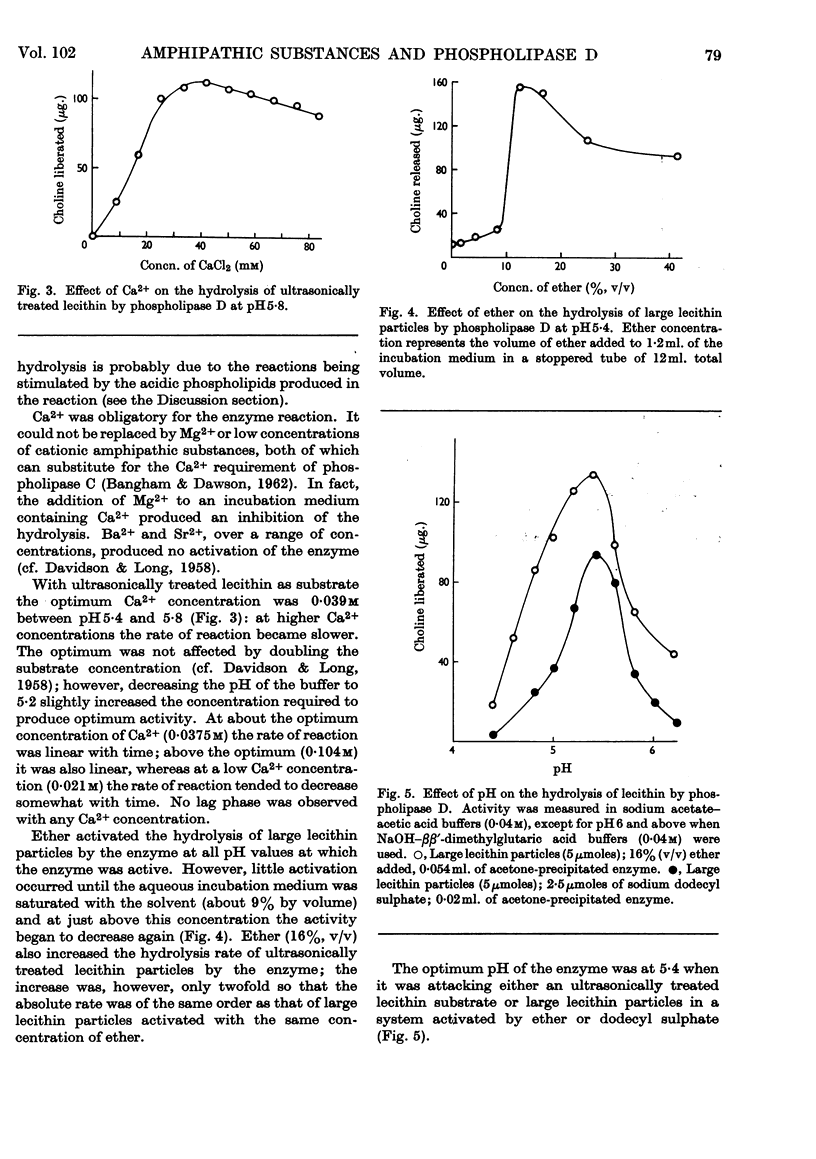



Selected References
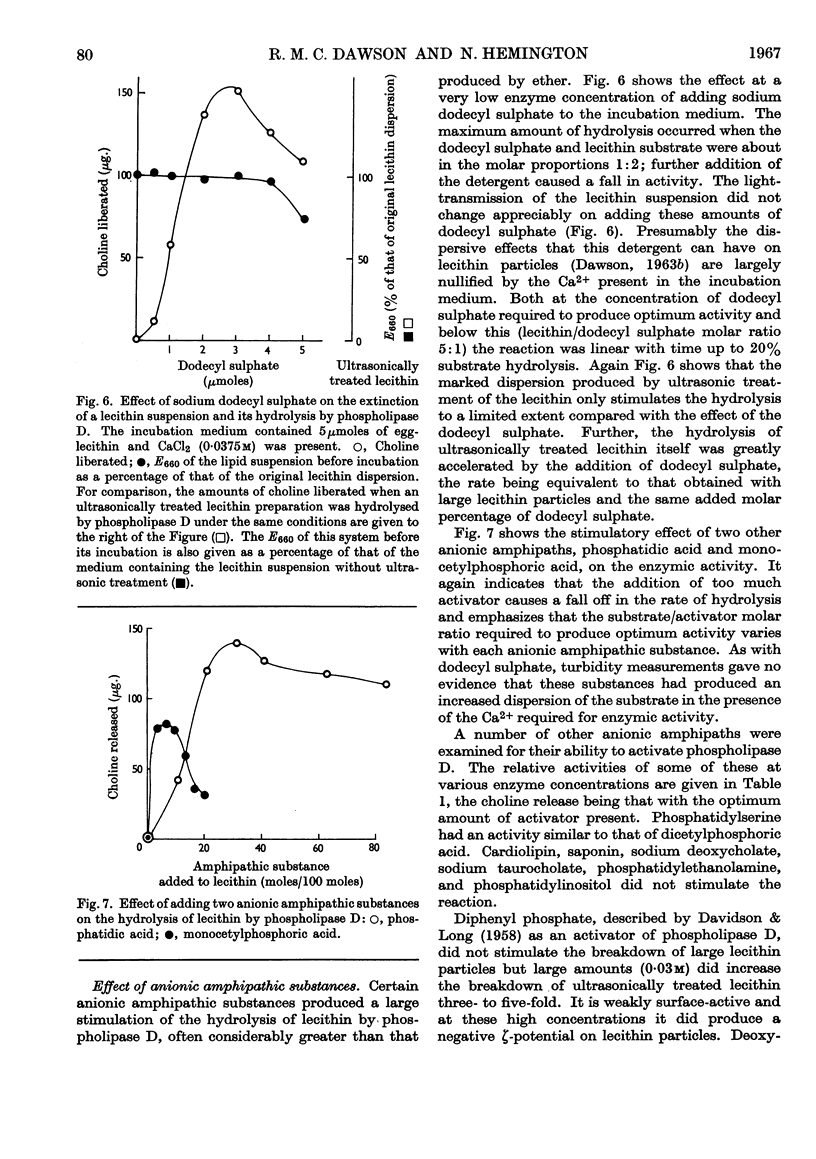
These references are in PubMed. This may not be the complete list of references from this article.
- APPLETON H. D., LA DU B. N., Jr, LEVY B., STEELE J. M., BRODIE B. B. A chemical method for the determination of free choline in plasma. J Biol Chem. 1953 Dec;205(2):803–813. [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. Electrokinetic requirements for the reaction between Cl. perfringens alpha-toxin (phospholipase C) and phospholipid substrates. Biochim Biophys Acta. 1962 May 7;59:103–115. doi: 10.1016/0006-3002(62)90701-1. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. The relation between the activity of a lecithinase and the electrophoretic charge of the substrate. Biochem J. 1959 Jul;72:486–492. doi: 10.1042/bj0720486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., HEARD D. H., FLEMANS R., SEAMAN G. V. An apparatus for microelectrophoresis of small particles. Nature. 1958 Sep 6;182(4636):642–644. doi: 10.1038/182642a0. [DOI] [PubMed] [Google Scholar]
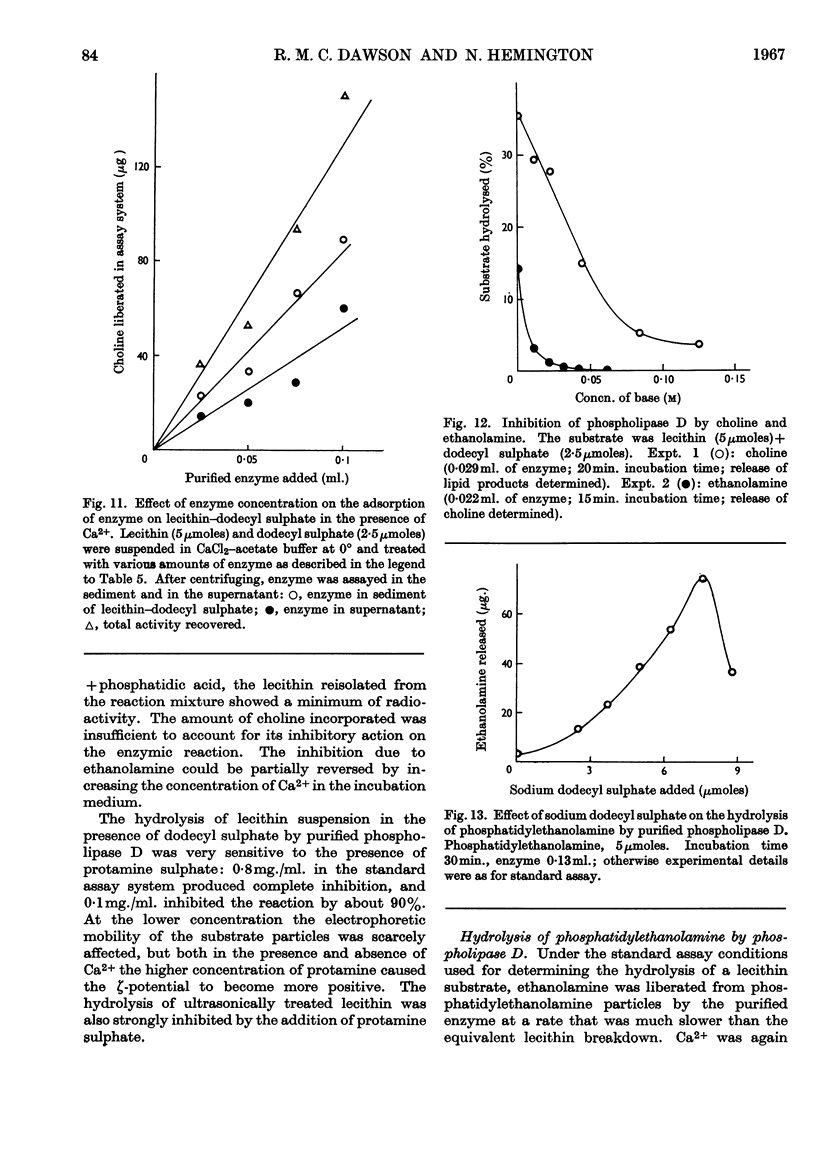
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. Liver glycerylphosphorylcholine diesterase. Biochem J. 1956 Apr;62(4):689–693. doi: 10.1042/bj0620689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EINSET E., CLARK W. L. The enzymatically catalyzed release of choline from lecithin. J Biol Chem. 1958 Apr;231(2):703–715. [PubMed] [Google Scholar]
- KATES M. Effects of solvents and surface-active agents on plastid phosphatidase C activity. Can J Biochem Physiol. 1957 Feb;35(2):127–142. [PubMed] [Google Scholar]
- SAUNDERS L., PERRIN J., GAMMACK D. Ultrasonic irradiation of some phospholipid sols. J Pharm Pharmacol. 1962 Sep;14:567–572. doi: 10.1111/j.2042-7158.1962.tb11141.x. [DOI] [PubMed] [Google Scholar]
- TOOKEY H. L., BALLS A. K. Plant phospholipase D. I. Studies on cottonseed and cabbage phospholipase D. J Biol Chem. 1956 Jan;218(1):213–224. [PubMed] [Google Scholar]
- WEISS H., SPIEGEL H. E., TITUS E. Isolation of an activator for phospholipase D. Nature. 1959 May 16;183(4672):1393–1394. doi: 10.1038/1831393a0. [DOI] [PubMed] [Google Scholar]


