Abstract
We investigated the contribution of multidrug resistance-associated protein 2 (MRP2) to the secretory transport of grepafloxacin and compared its functional role with that of P-glycoprotein (P-gp) by using Sprague-Dawley rats (SDRs) and Eisai hyperbilirubinemic rats (EHBRs), in which MRP2 is hereditarily defective. In intestinal tissue from SDRs mounted in Ussing chambers, the level of transport in the direction from the serosal layer to the mucosal layer was twofold greater than that in the direction from the mucosal layer to the serosal layer. This secretory transport of grepafloxacin was diminished by both probenecid, an MRP2 inhibitor, and cyclosporine, a P-gp inhibitor. In intestinal tissue from EHBRs, the secretory transport of grepafloxacin was lower than that in intestinal tissue from SDRs and was inhibited by cyclosporine but not by probenecid. The absorption of grepafloxacin from intestinal loops in SDRs was in the order of duodenum > jejunum > ileum and was increased by cyclosporine but not by probenecid. The absorption in EHBRs was not higher than that in SDRs. The intestinal secretory clearance in SDRs after intravenous administration of grepafloxacin was shown to be greater for the ileum than for the duodenum, which is in good agreement with the previously reported regional expression profile of MRP2 mRNA. The intestinal secretory clearance was lower in EHBRs than in SDRs. Accordingly, in addition to P-gp, MRP2 might play a role in the secretory transport of grepafloxacin. The function of MRP2 in facilitating grepafloxacin transport in the secretory direction is more pronounced both in vitro and in vivo, while the restriction of entry from the lumen into the cell by MRP2 seems to be negligible, compared with that by P-gp, in the case of grepafloxacin.
Both absorptive and secretory transporters in the intestine influence the oral bioavailabilities of drugs. Several absorptive transporters have been cloned, and their characterization has contributed to our understanding of the mechanisms of drug absorption (31). In addition, intestinal secretory transport systems have been extensively studied since the efflux transporters are likely to function as an absorption barrier, resulting in decreased bioavailabilities of drugs (2, 13, 30, 32).
Quinolone antimicrobial drugs are widely used due to their considerable oral bioavailabilities with limited entry into the brain (16, 21, 29). Despite the considerable bioavailabilities of quinolones (e.g., 72% for grepafloxacin) in vivo (27), secretory transport of quinolones in Caco-2 cells has been reported (4, 5, 8, 9, 33). We have also demonstrated that grepafloxacin transport is directed by the secretory transport system in Caco-2 cells and rat intestinal tissue and that P-glycoprotein (P-gp) is partially responsible for the secretion of grepafloxacin (23).
Among the transporters involved in secretion, P-gp has been the most investigated, and it has been suggested that it restricts the entry of several beta-blockers in rats (30) and Caco-2 cells (17), to restrict the entry of methylprednisolone in the rat small intestine (25), and to regulate the absorption of azasetron in vitro (28). As for the contribution of P-gp to quinolone transport, sparfloxacin secretion in Caco-2 cells was inhibited by verapamil, suggesting a contribution of P-gp (3). Using a P-gp-overexpressing cell line, Ito et al. (15) demonstrated that grepafloxacin is a substrate for P-gp. In vivo, P-gp partially restricts the entry of HSR-903, another quinolone, and grepafloxacin into the brain at the blood-brain barrier (21, 29). In the intestine, we have also demonstrated, using mdr1a and mdr1b gene-deficient mice, that P-gp partially functions as an efflux transporter for grepafloxacin and that anion-sensitive transport systems may be involved in secretory transport (23).
Involvement of a secretory transporter(s) other than P-gp has been found in studies with a Ussing chamber and Caco-2 cells: cefazolin and phenol red (24), calcein (6), and p-aminohippuric acid (22) showed secretory transport. Multidrug resistance-associated protein 2 (MRP2) is expressed significantly in the small intestines of Sprague-Dawley rats (SDRs) (14). While P-gp mainly transports neutral or cationic drugs, MRP2 may play a role in the efflux of anionic compounds in the intestine. Gotoh et al. (7) demonstrated that MRP2a contributes to the intestinal secretion of 2,4-dinitrophenyl-S-glutathione with SDRs and Eisai hyperbilirubinemic rats (EHBRs), in which MRP2 is hereditarily defective. Furthermore, Hirohashi et al. (10) suggested the ATP-dependent transport of conjugated metabolites by MRP2 in brush border membrane vesicles from Caco-2 cells.
The biliary clearance of grepafloxacin (18), HSR-903 (20), or sparfloxacin (1) is greater than that of other quinolones. A study with EHBRs demonstrated that grepafloxacin and its metabolites are secreted into the bile predominantly via MRP2 (26). Since grepafloxacin exists in the zwitterionic form at physiological pH, it can be transported as an anionic compound via organic anion transporters.
In the present study, using SDRs and EHBRs, we investigated whether MRP2 contributes to the secretion of grepafloxacin in the rat intestine. We also examined the functional roles of P-gp and MRP2 in grepafloxacin secretion in the small intestine.
MATERIALS AND METHODS
Chemicals.
[14C]grepafloxacin and unlabeled grepafloxacin were supplied by Otsuka Pharmaceutical Co., Ltd. (Tokushima, Japan). All other reagents were commercial products of reagent grade and were used without further purification.
Animals.
SDRs and EHBRs were purchased from Japan SLC (Hamamatsu, Japan). Studies with animals were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals in Takara-machi Campus of Kanazawa University.
Transport experiments by the Ussing-type chamber method.
Rat intestinal tissue sheets were prepared as described previously (22, 23, 29). The tissue sheets, consisting of the mucosa and most of the muscularis mucosa, were mounted vertically in an Ussing-type chamber that provided an exposed area of 0.5 cm2. The volume of bathing solution on each side was 5 ml, and the temperature was maintained at 37°C. The test solution consisted of 128 mM NaCl, 5.1 mM KCl, 1.4 mM CaCl2, 1.3 mM MgSO4, 21 mM NaHCO3, 1.3 mM KH2PO4, 10 mM NaH2PO4, and 5 mM glucose at pH 7.4 or 6.0 and was gassed with 95% O2–5% CO2 before and during the transport experiments. In the inhibition study, modulators were added on the same side to which the substrate was added.
Intestinal absorption study by loop method.
Rats were anesthetized by intraperitoneal administration of pentobarbital (50 mg/kg of body weight), the intestine was exposed by making a midline abdominal incision, and the bile duct was cannulated to collect the bile. Ten-centimeter closed loops of duodenum and ileum were prepared by ligation at both ends after clearing of the gut by slowly passing warmed isotonic 2-(N-morpholino)ethanesulfonic acid (MES) buffer (5 mM KCl, 100 mM NaCl, 10 mM MES, 85 mM mannitol, polyethylene glycol 0.01% [pH 6.4]; osmolarity, 290 mosM/kg) until the effluent became clear and expelling the remaining solution with air pumped with a syringe. Five hundred microliters of isotonic MES buffer, including unlabeled grepafloxacin (10 μM), was administered into the loops as a bolus. The animal was kept on a warm plate at 37°C. After 15 min, the solution in the loop was collected and the loop was rinsed with isotonic MES buffer to give a total volume of 5 ml. The concentration of grepafloxacin in the effluent was measured by high-pressure liquid chromatography (HPLC) to estimate the remaining amount of grepafloxacin. The fraction of absorbed grepafloxacin was estimated from the difference between the dose administered and the amount remaining in the loops.
Intestinal secretion study by in situ loop method.
Twenty-centimeter closed loops of duodenum and ileum were prepared by the same procedure described above. Each loop was filled with 2.0 ml of isotonic MES buffer. After the preparation procedures were completed, the rat was kept on a warm plate at 37°C for a 10-min recovery period. [14C]grepafloxacin was given intravenously (i.v.) into the femoral vein of each animal at a dose of 1 μCi (34 nmol). Blood was collected from the right carotid artery via a cannula at designated times, and the plasma was prepared by centrifugation. One hour after administration, the solution in the loop was collected and the loop was rinsed with ice-cold isotonic MES buffer to make a total volume of 5 ml.
Analytical methods.
To assay the radioactivity, all samples were transferred into counting vials, mixed with scintillation fluid (Cleasol I; Nacalai Tesque, Kyoto, Japan), and quantified in a liquid scintillation counter (Aloka, Tokyo, Japan). Nonradioactive grepafloxacin was used in the Ussing chamber method and the in situ loop absorption method, and the amount was measured by HPLC. The HPLC system consisted of a constant-flow pump (980-PU; Japan Spectroscopic Co., Tokyo, Japan), a fluorescence detector (RF-550; Shimadzu Co., Kyoto, Japan), an integrator (Chromatopac CR3A; Shimadzu Co.), and an automatic sample injector (AS-950; Japan Spectroscopic Co.). A TSK gel column (ODS-80Ts; 150 by 4.6 mm [inner diameter]; Tosoh, Tokyo, Japan) was used as in the analytical column. The excitation and emission wavelengths were 282 and 448 nm, respectively, and the column was heated at 50°C. The mobile phase consisted of acetonitrile-water-phosphoric acid (25:75:0.1; vol/vol/vol), and the flow rate was 0.8 ml min−1. The assay was shown to be linear over the concentration range used in the present study.
Data analysis.
Transport was estimated in terms of permeation (in microliters per square centimeter), obtained by dividing the amount transported (in micromoles per square centimeter) by the initial concentration of test compound on the donor side (in micromoles per microliter). The permeability coefficient (in microliters per square centimeter per minute) was obtained from the slope of the linear portion of the plots of permeation against time (minutes). All data are expressed as means ± standard errors (SEs), and statistical analysis was performed by Student’s t test. A difference between means was considered significant when the P value was less than 0.05.
RESULTS
Grepafloxacin transport across rat small intestinal tissue.
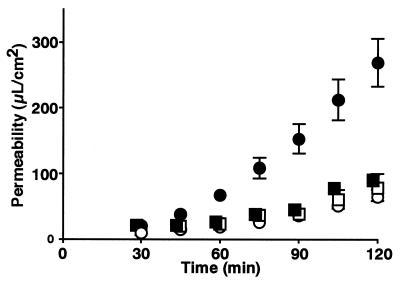
We previously showed that grepafloxacin permeation was directed by the secretory transport system in SDR ileal tissue by the Ussing chamber method (23). In order to characterize further the secretory transport systems of grepafloxacin in the intestine, an inhibition study was performed (Table 1). The addition of cyclosporine showed a tendency to increase permeability in the direction from the mucosal layer to the serosal layer (m-s) and significantly decreased the permeability in the direction from the serosal layer to the mucosal layer (s-m). The addition of probenecid caused similar changes in grepafloxacin transport. We then isolated intestinal tissue from EHBRs, which lack a functional mrp2 gene. Although the m-s permeability coefficient for EHBRs was comparable to that for SDRs, the s-m permeability coefficient for EHBRs was significantly smaller than that for SDRs (Fig. 1), resulting in decreases in net secretion and the secretory ratio in EHBRs (Table 2). In EHBRs, cyclosporine significantly increased the m-s permeability coefficient, resulting in a decrease in net secretion from 0.404 ± 0.195 to 0.087 ± 0.108 μl/cm2/min. Although probenecid significantly increased both the m-s and the s-m permeability coefficients, it did not cause any significant change in net secretion or the secretory ratio in EHBRs. The combination of the two modulators resulted in a significant change in s-m permeability, similar to that observed in the presence of cyclosporine alone (Table 2).
TABLE 1.
Inhibitory effects of P-gp and MRP2 modulators on grepafloxacin transport in isolated SDR intestinal tissue mounted in an Ussing chambera
| Treatment | Permeability coefficient (μl/cm2/min)
|
Net secretion (μl/cm2/min) [(s-m) − (m-s)] | Secretory ratio [(s-m)/(m-s)] | |
|---|---|---|---|---|
| m-s | s-m | |||
| Control | 0.759 ± 0.100 | 3.25 ± 0.48 | 2.49 ± 0.40 | 4.32 ± 0.36 |
| Cyclosporine (10 μM) | 1.03 ± 0.14 | 1.90 ± 0.52b | 0.861 ± 0.266b | 2.03 ± 0.42b |
| Probenecid (1 mM) | 1.13 ± 0.18 | 1.80 ± 0.20b | 0.667 ± 0.185b | 1.75 ± 0.24b |
| Cyclosporine (10 μM) + probenecid (1 mM) | 0.515 ± 0.029 | 0.905 ± 0.035b | 0.390 ± 0.055b | 1.80 ± 0.14b |
The time course of grepafloxacin (10 μM) transport across the intestinal tissue was evaluated. The modulators were added to the donor solution. The experimental solution was adjusted to pH 7.4, and the temperature was maintained at 37°C.
Significantly different from control value by Student’s t test (P < 0.05).
FIG. 1.
Grepafloxacin transport in intestinal isolated tissue from SDRs and EHBRs and mounted in Ussing chambers. The transport of grepafloxacin (1 μM) across the intestinal tissue was evaluated from the time course of transport by the Ussing chamber method. The experimental solution was adjusted to pH 7.4, and the temperature was maintained at 37°C. Circles and squares, transport in SDRs and EHBRs, respectively; open and closed symbols, m-s and s-m, respectively. Each point represents the mean ± SE of four to seven experiments.
TABLE 2.
Grepafloxacin transport in EHBR intestinal tissue mounted in an Ussing chamber and inhibitory effects of P-gp and MRP2 modulatorsa
| Rat strain and treatment | Permeability coefficient (μl/cm2/min)
|
Net secretion (μl/cm2/min) [(s-m) − (m-s)] | Secretory ratio [(s-m)/(m-s)] | |
|---|---|---|---|---|
| m-s | s-m | |||
| SDR (control) | 0.759 ± 0.100 | 3.25 ± 0.48 | 2.49 ± 0.40 | 4.32 ± 0.36 |
| EHBR | ||||
| None | 0.652 ± 0.127 | 1.06 ± 0.17b | 0.404 ± 0.195b | 1.77 ± 0.37b |
| Cyclosporine (10 μM) | 1.26 ± 0.28c | 1.34 ± 0.28 | 0.087 ± 0.108 | 1.11 ± 0.11c |
| Probenecid (1 mM) | 1.12 ± 0.13c | 2.07 ± 0.33c | 0.951 ± 0.429 | 2.04 ± 0.59 |
| Cyclosporine (10 μM) + probenecid (1 mM) | 1.68 ± 0.09c | 1.19 ± 0.14 | −0.485 ± 0.208c | 0.727 ± 0.123c |
The time course of grepafloxacin (10 μM) transport across the intestinal tissue was evaluated. The modulators were added to the donor solution. The experimental solution was adjusted to pH 7.4, and the temperature was maintained at 37°C.
Significantly different from the value for SDRs (control) by Student’s t test (P < 0.05).
Significantly different from the value for EHBRs by Student’s t test (P < 0.05).
Grepafloxacin absorption from rat small intestinal loops.
We examined the absorption of grepafloxacin from the intestine by the in situ closed-loop method in SDRs and EHBRs (Table 3). In SDRs, the absorption from the duodenum was very fast, and 77.0% of the dose disappeared in 15 min. The disappearances of the doses from the jejunum and ileum were 41.8 and 40.5%, respectively, being significantly lower than that from the duodenum. Cyclosporine significantly increased the level of absorption from the ileum, and probenecid significantly decreased the level of absorption from the duodenum. In EHBRs, the disappearance from the duodenum was less than that in SDRs, but no difference in the disappearance of the doses from the jejunum or the ileum was observed between the two strains.
TABLE 3.
Grepafloxacin absorption from intestine by in situ loop method in SDRs and EHBRs and inhibitory effects of P-gp and MRP2 modulatorsa
| Strain and inhibitor | % Absorption
|
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| SDR | |||
| None | 77.0 ± 3.6 | 41.8 ± 4.0 | 40.5 ± 4.3 |
| Cyclosporine (5 μM) | 69.7 ± 5.2 | 50.8 ± 4.6 | 59.2 ± 2.5b |
| Probenecid (1 mM) | 57.2 ± 2.0b | 44.2 ± 4.1 | 45.2 ± 1.4 |
| EHBR, none | 59.2 ± 6.2b | 39.8 ± 8.1 | 45.8 ± 6.0 |
In the loop absorption experiment, 0.5 ml of isotonic MES buffer containing 10 μM grepafloxacin (pH 6.0) was administered into a 10-cm loop of duodenum, jejunum, or ileum. The luminal fluid in each loop was collected 15 min after administration. The fraction absorbed was estimated as the difference between the dose administered and the amount remaining in the loops. Each datum represents the mean ± SE of four to six experiments.
Significantly different from the corresponding value for SDRs by Student’s t test (P < 0.05).
Grepafloxacin secretion into intestinal luminal loops and biliary secretion in rats.
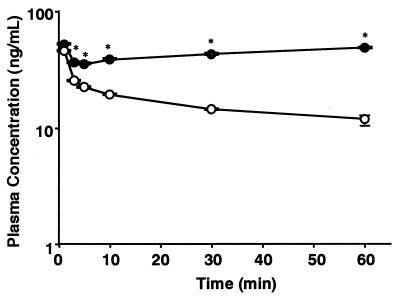
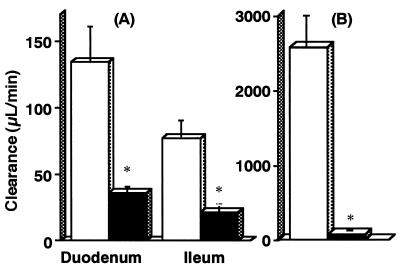
We investigated whether grepafloxacin injected i.v. is secreted in the intestine. Figure 2 shows the time course of the concentration of [14C]grepafloxacin in plasma after i.v. administration of 34 nmol to SDRs and EHBRs. The radioactivity in the plasma of EHBRs was significantly higher than that in the plasma of SDRs after 2 min, resulting in differences in the pharmacokinetic parameters (Table 4). Since the radioactivity in the plasma of EHBRs increased from 10 to 60 min after i.v. administration, the elimination rate constant, total clearance from plasma, and volume of distribution at steady state could not be estimated. As shown in Fig. 3, the duodenal and ileal secretory clearances in SDRs were 134 ± 26 and 77 ± 13 μl/min, respectively. In EHBRs, those values were 36 ± 5 and 21 ± 6 μl/min, respectively, being significantly smaller than those in SDRs. The biliary clearance in EHBRs was also significantly lower than that in SDRs.
FIG. 2.
Time courses of the concentrations of [14C]grepafloxacin in plasma after i.v. administration to SDRs and EHBRs. [14C]grepafloxacin (34 nmol) dissolved in saline solution was administered intravenously to SDRs (open circles) or EHBRs (closed circles). Serial blood samples were collected from the carotid artery of individual rats via a cannula at designated time intervals over the experimental period. Each point represents the mean ± standard error of the mean for four to six animals. *, significant difference between SDRs and EHBRs by Student’s t test (P < 0.05).
TABLE 4.
Pharmacokinetic parameters in the secretory loop experiment after i.v. administration of grepafloxacina
| Strain | kel (min−1) | AUC (μg · min/ml) | CL (ml/min) | MRT (min) | VSS (ml) |
|---|---|---|---|---|---|
| SDR | 0.014 ± 0.001 | 1.01 ± 0.080 | 7.09 ± 0.83 | 24.2 ± 0.7 | 486 ± 23 |
| EHBR | —b | 2.60 ± 0.19c | —b | 31.6 ± 0.3c | —b |
Pharmacokinetic parameters were estimated by model-independent moment analysis with WinNonlin software. Abbreviations: kel, linear terminal elimination rate constant; AUC, area under the plasma concentration-time curve from time zero to 60 min; CL, total clearance from plasma; MRT, mean residence time from time zero to infinity: VdSS, volume of distribution at steady state.
Since the radioactivity increased in the plasma of EHBRs from 10 to 60 min after i.v. administration, the values for the parameters could not be estimated.
Significant difference between SDRs and EHBRs by Student’s t test (P < 0.05).
FIG. 3.
Intestinal (A) and biliary (B) clearances of [14C]grepafloxacin and its metabolites after i.v. administration in rats. Five hundred microliters of isotonic MES buffer was administered into 10-cm loops of duodenum and ileum, and then [14C]grepafloxacin was administered intravenously at a dose of 34 nmol per animal. Bile was collected via a cannula for 60 min, and at the end of the experiment, the intestinal loops were removed and the solution in each loop was collected. The clearance values were estimated by dividing the [14C]grepafloxacin recovered in the intestinal loops or bile by the area under the plasma concentration-time curve from time zero to 60 min. Open and closed columns, means ± standard errors of the means for SDRs and EHBRs, respectively; *, significant difference between SDRs and EHBRs by Student’s t test (P < 0.05).
DISCUSSION
In the present study, we examined the mechanisms involved in the secretion of grepafloxacin into the rat intestinal lumen by using in vitro and in vivo experimental methods.
There are several reports that quinolone antimicrobials are secreted into the intestine (4, 5, 8, 9). We have recently reported that grepafloxacin is transported preferentially in the secretory direction in Caco-2 cell monolayers, and we demonstrated the contribution of P-gp in vivo by using P-gp gene-deficient mice (23). We further suggested that the secretion of grepafloxacin is also mediated by transport systems other than those involving P-gp. Since most of the grepafloxacin molecules exist in zwitterionic form at physiological pH and secretory transport was diminished by the addition of probenecid to Caco-2 cells, the involvement of an anion transport system(s) was suggested. Sasabe et al. (26) have reported that grepafloxacin is secreted by MRP2 at the canalicular membrane in the liver. MRP2 was also reported to be expressed in the intestine, and it was demonstrated that MRP2 transports glucuronide conjugates in the secretory direction in the intestine (7). Furthermore, the distribution of several quinolones including grepafloxacin in the brain is restricted by the operation of multiple efflux transporters, such as MRP1, P-gp, and a 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid and bicarbonate ion-sensitive anion-exchange transporter (21, 29). Therefore, we hypothesized that grepafloxacin is also secreted by MRP2 in the intestine. To focus closely on the contribution of MRP2 to the secretory transport of grepafloxacin in the intestine, we used EHBRs, in which MRP2 is hereditarily defective, and examined the roles of MRP2 and P-gp in grepafloxacin transport in the intestine by means of an inhibition study.
First, we performed an inhibition study with modulators of MRP2 and P-gp (probenecid and cyclosporine, respectively) to characterize the secretion of grepafloxacin in isolated intestinal tissue mounted in an Ussing chamber. We have previously shown by the Ussing chamber method that grepafloxacin permeation is directed by the secretory transport system in SDR ileal tissue (23). In SDR intestinal tissue, the secretory transport ratio, calculated as the s-m permeability coefficient divided by the m-s permeability coefficient, decreased upon the addition of probenecid, cyclosporine, or the combination of the two. The concentration of cyclosporine (5 to 10 μM) is enough to inhibit P-gp (11), supporting the idea that P-gp contributes to the secretion of grepafloxacin in the intestine (23). However, probenecid (1 mM) probably inhibits not only MRP2 but also other anionic transport systems. Therefore, to confirm that MRP2 is involved in the secretory transport of grepafloxacin, intestinal tissue from EHBRs was used. Since MRP2 is hereditarily defective in EHBRs and its mRNA is undetectable by Northern blotting (7), the contribution of MRP2 can be estimated by comparing the transport activities in SDRs and EHBRs. The value of the s-m permeability coefficient in EHBRs was significantly smaller than that in SDRs, resulting in decreases in net secretion and the secretory ratio. This result confirmed that MRP2 contributes to the secretory transport of grepafloxacin in isolated rat intestinal tissue. However, secretory transport was still observed in EHBRs. This secretory transport disappeared when cyclosporine or the combination of cyclosporine and probenecid was added, while no statistically significant difference in net secretion or the secretory ratio was detected when only probenecid was added. This result suggests that the decrease in the secretory transport of grepafloxacin in SDRs caused by probenecid is the result of inhibition of MRP2, while EHBRs still retained secretory activity, probably due to P-gp.
It is of great importance to investigate the significance of MRP2 in the intestine in vivo. We have already demonstrated, using mdr1a gene-defective mice, that P-gp mediated the secretion of grepafloxacin into the intestinal lumen after i.v. administration and that it restricted absorption from the lumen (23). In the present study, we compared intestinal clearance after the i.v. administration of grepafloxacin and the disappearance of grepafloxacin from the lumen by the closed-loop method in SDRs and EHBRs to determine whether MRP2 contributes to secretion as well as to the absorption barrier for grepafloxacin in the intestine. In the secretion experiment, [14C]grepafloxacin was administered i.v. to SDRs and EHBRs, and the intestinal secretion was examined in relation to the biliary excretion in vivo. In SDRs, 20.2% ± 2.8% of the total radioactivity was recovered in bile 60 min after administration, while in EHBRs only 1.71% ± 0.50% of the total radioactivity was recovered. It has been reported that the major route of excretion of grepafloxacin and its metabolites is via biliary excretion and that MRP2 on the canalicular membrane contributes significantly to the excretion (26); therefore, it was not unexpected that the profiles of [14C]grepafloxacin (and its metabolites) in plasma after i.v. administration of grepafloxacin were different between SDRs and EHBRs, leading to a 2.5-fold increase in the area under the plasma concentration-time curve from time zero to 60 min in EHBRs (Table 4). The secretory clearance in the duodenum was twofold greater than that in the ileum. This result is also in good agreement with the heterogeneous distribution of MRP2 expression in the intestine (which is greater in the duodenum than in the ileum) (19). Moreover, the values of intestinal secretory clearances were significantly decreased in EHBRs compared with those in SDRs, and the intestinal regional difference became less marked (Fig. 3). This result strongly indicates that MRP2 is involved as an efflux transporter in the secretory transport of grepafloxacin. In the present study, however, we measured the total radioactivity recovered in the intestinal loop. Sixty minutes after i.v. administration of [14C]grepafloxacin, the proportions of unchanged [14C]grepafloxacin in plasma were about 80 and 55% in SDRs and EHBRs, respectively. However, the recovery in the intestinal lumen after i.v. administration was too small to analyze the relative ratio of unchanged and glucuronidated grepafloxacin. Therefore, the intestinal secretory clearance value in the present study may be the sum of the intestinal clearance values for both the parent grepafloxacin and its metabolites. An additional point to be considered may be the functional expression of other transporters such as MRP3 were present in EHBRs, since MRP3 was suggested to be induced in EHBRs. If grepafloxacin and its metabolites are transported by MRP3, that may affect the intestinal disposition.
In the intestine, an increase in absorption is expected if secretory transport is inhibited. As expected, cyclosporine increased the absorption in the ileum. This is in good agreement with a report demonstrating that the expression of P-gp is higher in the lower region than in the upper region of the intestine (12). However, probenecid did not increase the level of absorption and even decreased the level of absorption in the duodenum. These results may indicate that both MRP2 and P-gp are responsible for the intestinal secretion of grepafloxacin into the lumen, and P-gp likely limits the absorption from the intestine, while the contribution of MRP2 as an absorption barrier is less significant. That is, P-gp contributes to both the limitation of entry and the facilitation of excretion of grepafloxacin, whereas MRP2 mainly functions in the facilitation of excretion into the intestinal lumen. This may be accounted for by the higher affinity of grepafloxacin glucuronide to MRP2 than that of unchanged grepafloxacin. We set the grepafloxacin concentration at 10 μM for the loop absorption experiment. This concentration might cause the saturation of intestinal MRP2 after oral administration. Therefore, it could also be possible that the contribution of MRP2 as an absorptive barrier was not observed in this experiment.
Yamaguchi et al. (33) recently reported that probenecid does not affect the transcellular transport of [14C]grepafloxacin in Caco-2 cells and suggested that the contribution of MRP2 to the intestinal secretion of grepafloxacin may be small. However, they examined the inhibition effect of probenecid on transport in the secretory direction alone. We also reported on the involvement of multiple efflux transport systems for [14C]grepafloxacin in Caco-2 cells (23). In that study, we also failed to detect a decrease in grepafloxacin transport in the secretory direction, but absorptive transport was increased in the presence of probenecid. The reason for the apparent discrepancy in the inhibitory effect of probenecid on grepafloxacin transport between the rat intestine and Caco-2 cells is not clear. However, we have preliminary data indicating that an unstirred water layer effect may decrease the inhibitory effect on secretory transport because of a high apparent membrane permeability. The observation that absorptive transport of grepafloxacin was increased by probenecid in Caco-2 cells suggests that the contribution of MRP2 may be lower in the rat intestine than in Caco-2 cells. Although the quantitative contributions of the two secretory transporters are not clear, it was demonstrated that MRP2 functions as an efflux transporter for grepafloxacin in the intestine both in vivo and in vitro by using MRP2-deficient EHBRs.
In conclusion, MRP2, in addition to P-gp, plays a role in the secretory transport of grepafloxacin. In contrast to P-gp, the restriction of entry from the lumen into the cell by MRP2 seems to be negligible in the case of grepafloxacin. Discrimination of grepafloxacin and its conjugates may be needed in order to reach a better understanding of the intestinal transport of quinolone antimicrobials.
REFERENCES
- 1.Akiyama, H., M. Koike, K. Kyuushiki, T. Suzuki, N. Kusumoto, S. Morita, and M. Odomi. 1995. Pharmacokinetics of grepafloxacin. IV. Metabolism after oral administration of grepafloxacin in rats, monkeys and humans. Chemotherapy (Tokyo) 43(Suppl. 1):131–149. [Google Scholar]
- 2.Arimori, K., and M. Nakano. 1998. Drug exsorption from blood into the gastrointestinal tract. Pharm. Res. 15:371–376. [DOI] [PubMed] [Google Scholar]
- 3.Cormet-Boyaka, E., J. F. Huneau, A. Mordrelle, P. N. Boyaka, C. Carbon, E. Rubinstein, and D. Tome. 1997. Secretion of sparfloxacin from the human intestinal Caco-2 cell line is altered by P-glycoprotein inhibitors. Antimicrob. Agents Chemother. 42:2607–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormet-Boyaka, E., J. F. Huneau, M. Bouras, C. Carbon, E. Rubinstein, and D. Tome. 1997. Evidence for a passive diffusion mechanism for sparfloxacin uptake at the brush-border membrane of the human intestinal cell-line Caco-2. J. Pharm. Sci. 86:33–36. [DOI] [PubMed] [Google Scholar]
- 5.Dautrey, S., K. Felice, A. Petiet, B. Lacour, C. Carbon, and R. Farinotti. 1999. Active intestinal elimination of ciprofloxacin in rats: modulation by different substrates. Br. J. Pharmacol. 127:1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita, T., H. Yamada, M. Fukuzumi, A. Nishimaki, A. Yamamoto, and S. Muranishi. 1997. Calcein is excreted from the intestinal mucosal cell membrane by the active transport system. Life Sci. 60:307–313. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh, Y., H. Suzuki, S. Kinoshita, T. Hirohashi, Y. Kato, and Y. Sugiyama. 2000. Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J. Pharmacol. Exp. Ther. 292:433–439. [PubMed] [Google Scholar]
- 8.Griffiths, N. M., B. H. Hirst, and N. L. Simmons 1993. Active secretion of the fluoroquinolone ciprofloxacin by human intestinal epithelial Caco-2 cell layers. Br. J. Pharmacol. 108:575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths, N. M., B. H. Hirst, and N. L. Simmons. 1994. Active intestinal secretion of the fluoroquinolone antibacterials ciprofloxacin, norfloxacin and pefloxacin; a common secretory pathway? J. Pharmacol. Exp. Ther. 269:496–502. [PubMed] [Google Scholar]
- 10.Hirohashi, T., H. Suzuki, X. Y. Chu, I. Tamai, A. Tsuji, and Y. Sugiyama. 2000. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J. Pharmacol. Exp. Ther. 292:265–270. [PubMed] [Google Scholar]
- 11.Hollo, Z., L. Homolya, T. Hegedus, and B. Sarkadi. 1996. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 383:99–104. [DOI] [PubMed] [Google Scholar]
- 12.Hsing, S., Z. Gatmaitan, and I. M. Arias. 1992. The function of Gp170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology 102:879–885. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, J., and B. H. Hirst. 1997. Intestinal secretion of drugs. The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv. Drug Deliv. Rev. 25:129–157. [Google Scholar]
- 14.Ito, K., H. Suzuki, T. Hirohashi, K. Kume, T. Shimizu, and Y. Sugiyama. 1997. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am. J. Physiol. 272:G16–G22. [DOI] [PubMed] [Google Scholar]
- 15.Ito, T., I. Yano, K. Tanaka, and K. I. Inui. 1997. Transport of quinolone antibacterial drugs by human P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J. Pharmacol. Exp. Ther. 282:955–960. [PubMed] [Google Scholar]
- 16.Jaehde, U., M. W. Langemeijer, A. G. de Boer, and D. D. Breimer. 1992. Cerebrospinal fluid transport and disposition of the quinolones ciprofloxacin and pefloxacin in rats. J. Pharmacol. Exp. Ther. 263:1140–1146. [PubMed] [Google Scholar]
- 17.Karlsson, J., S. M. Kuo, J. Ziemniak, and P. Artursson. 1993. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. Br. J. Pharmacol. 110:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsunaga, Y., H. Miyazaki, Y. Oh-e, K. Nambu, H. Furukawa, K. Yoshida, and M. Hashimoto. 1991. Disposition and metabolism of 14C-sparfloxacin in the rat. Arzneim-Forsch./Drug Res. 41:747–759. [PubMed] [Google Scholar]
- 19.Mottino, A. D., T. Hoffman, L. Jennes, and M. Vore. 2000. Expression and localization of multidrug resistant protein Mrp2 in rat small intestine. J. Pharmacol. Exp. Ther. 293:717–723. [PubMed] [Google Scholar]
- 20.Murata, M., I. Tamai, Y. Sai, O. Nagata, H. Kato, Y. Sugiyama, and A. Tsuji. 1998. Hepatobiliary transport kinetics of HSR-903, a new quinolone antibacterial agent. Drug Metab. Dispos. 26:1113–1119. [PubMed] [Google Scholar]
- 21.Murata, M., I. Tamai, H. Kato, O. Nagata, and A. Tsuji. 1999. Efflux transport of a new quinolone antibacterial agent, HSR-903, across the blood-brain barrier. J. Pharmacol. Exp. Ther. 290:51–57. [PubMed] [Google Scholar]
- 22.Naruhashi, K., I. Tamai, Y. Sai, N. Suzuki, and A. Tsuji. 2001. Secretory transport of p-aminohippuric acid across intestinal epithelial cells in Caco-2 cells and isolated intestinal tissue. J. Pharm. Pharmacol. 53:73–81. [DOI] [PubMed] [Google Scholar]
- 23.Naruhashi, K., I. Tamai, N. Inoue, H. Muraoka, Y. Sai, N. Suzuki, and A. Tsuji. 2001. Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J. Pharm. Pharmacol. 53:699–709. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh, H., C. Gerard, and B. J. Aungst. 1996. The secretory intestinal transport of some beta-lactam antibiotics and anionic compounds: a mechanism contributing to poor oral absorption. J. Pharmacol. Exp. Ther. 278:205–211. [PubMed] [Google Scholar]
- 25.Saitoh, H., M. Hatakeyama, O. Eguchi, M. Oda, and M. Takada. 1998. Involvement of intestinal P-glycoprotein in the restricted absorption of methylprednisolone from rat small intestine. J. Pharm. Sci. 87:73–75. [DOI] [PubMed] [Google Scholar]
- 26.Sasabe, H., A. Tsuji, and Y. Sugiyama. 1998. Carrier-mediated mechanism for the biliary excretion of the quinolone antibiotic grepafloxacin and its glucuronide in rats. J. Pharmacol. Exp. Ther. 284:1033–1039. [PubMed] [Google Scholar]
- 27.Sorgel, F., U. Jaehde, K. Naber, and U. Stephan. 1989. Pharmacokinetic disposition of quinolones in human body fluids and tissues. Clin. Pharmacokinet. 16(Suppl. 1):5–24. [DOI] [PubMed] [Google Scholar]
- 28.Tamai, I., A. Saheki, R. Saitoh, Y. Sai, I. Yamada, and A. Tsuji. 1997. Nonlinear intestinal absorption of 5-hydroxytryptamine receptor antagonist caused by absorptive and secretory transporters. J. Pharmacol. Exp. Ther. 283:108–115. [PubMed] [Google Scholar]
- 29.Tamai, I., J. Yamashita, Y. Kido, A. Ohnari, Y. Sai, Y. Shima, K. Naruhashi, S. Koizumi, and A. Tsuji. 2000. Limited distribution of new quinolone antibacterial agents into brain caused by multiple efflux transporters at the blood-brain barrier. J. Pharmacol. Exp. Ther. 295:146–152. [PubMed] [Google Scholar]
- 30.Terao, T., E. Hisanaga, Y. Sai, I. Tamai, and A. Tsuji. 1996. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol. 48:1083–1089. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji, A., and I. Tamai. 1996. Carrier-mediated intestinal transport of drugs. Pharm. Res. 13:963–977. [DOI] [PubMed] [Google Scholar]
- 32.Wacher, V. J., J. A. Silverman, Y. Zhang, and L. Z. Benet. 1998. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J. Pharm. Sci. 87:1322–1330. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi, H., I. Yano, Y. Hashimoto, and K. I. Inui. 2000. Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 295:360–366. [PubMed] [Google Scholar]