Abstract
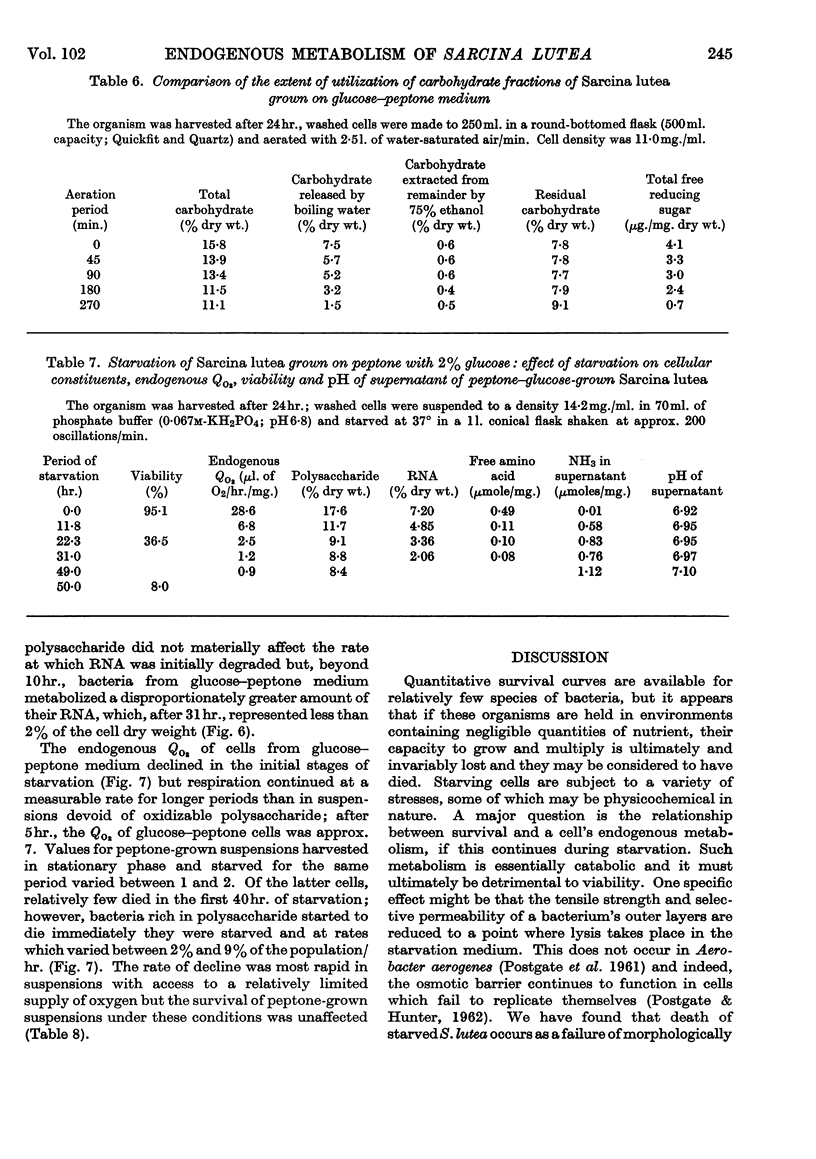
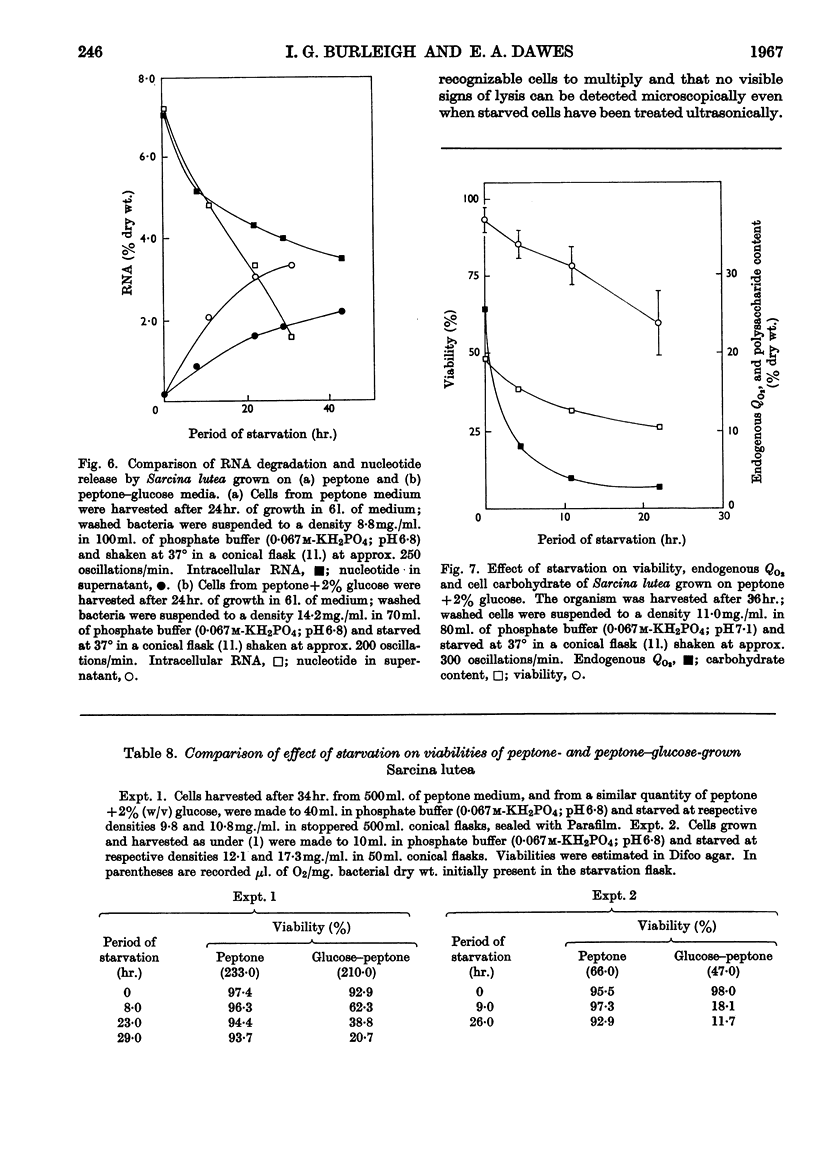
1. When washed suspensions of Sarcina lutea are starved aerobically in phosphate buffer at the growth temperature of 37°, the rate of endogenous oxygen consumption decreases to very low values after 10hr., although many of the cells survive for 40hr. If starvation is prolonged further, the bacteria die at a rate of approximately 1·5% of the initial viable population per hour. 2. Oxidation of intracellular free amino acids accounts for most of the observed endogenous oxygen uptake but RNA is also utilized and a portion of the component bases and pentose is degraded and presumably oxidized. Ammonia appears in the supernatant and some pentose and ultraviolet-absorbing nucleotide are released from the cells. DNA, protein and polysaccharide are not measurably degraded. 3. Survival can be correlated with the ability of aerobically starved bacteria to oxidize exogenous l-glutamate and glucose. When starved under nitrogen for 40hr. cells continue to oxidize their endogenous reserves at undiminished rates when transferred to aerobic conditions; on prolonging anaerobic starvation the rate of oxidation declines during the period of most rapid loss of viability. 4. In the presence of Mg2+, RNA degradation during aerobic starvation is almost completely suppressed without affecting the period for which the bacteria survive. 5. Cells grown in peptone supplemented with glucose accumulate reserves of polysaccharide which are metabolized in aerobic starvation, together with free amino acids. Ammonia is evolved and RNA is degraded to a greater extent than in peptone-grown suspensions. Bacteria rich in polysaccharide survive less well than those which are deficient in the polymer; the reason for this phenomenon has yet to be established. 6. In peptone medium, endogenous oxygen uptake and the concentration of intracellular free amino acids decline as growth progresses and they continue to decrease when the organism is held in stationary phase. Under the conditions used, the endogenous Qo2 and free amino acid pool of cells grown in peptone with 2% (w/v) glucose did not decline so markedly and the bacteria contained large amounts of polysaccharide at all stages of growth.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
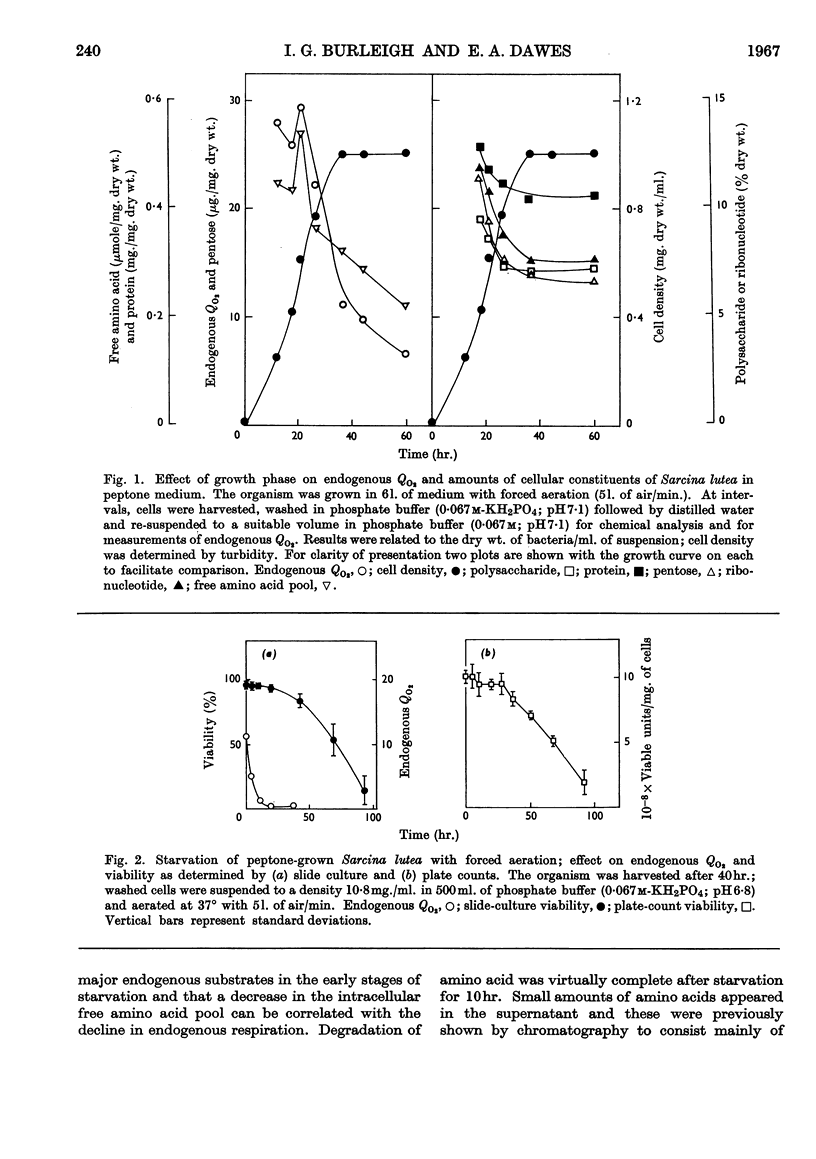
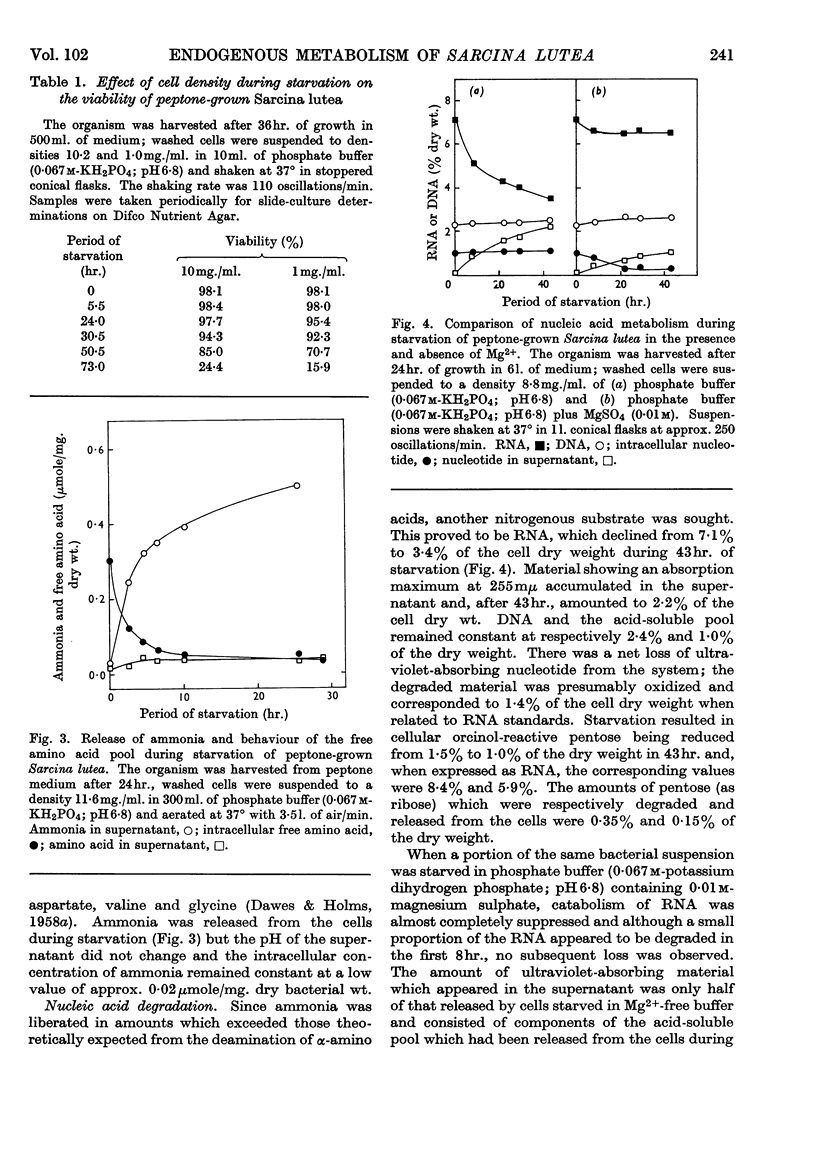
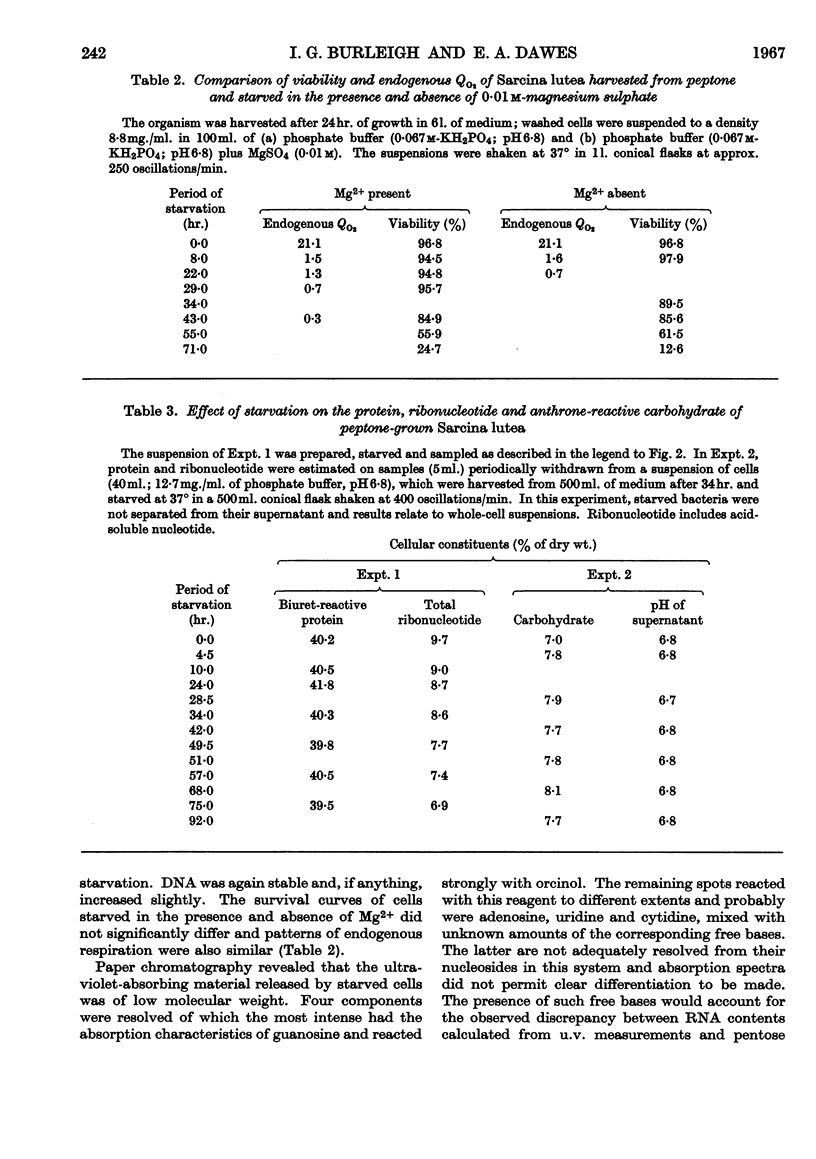
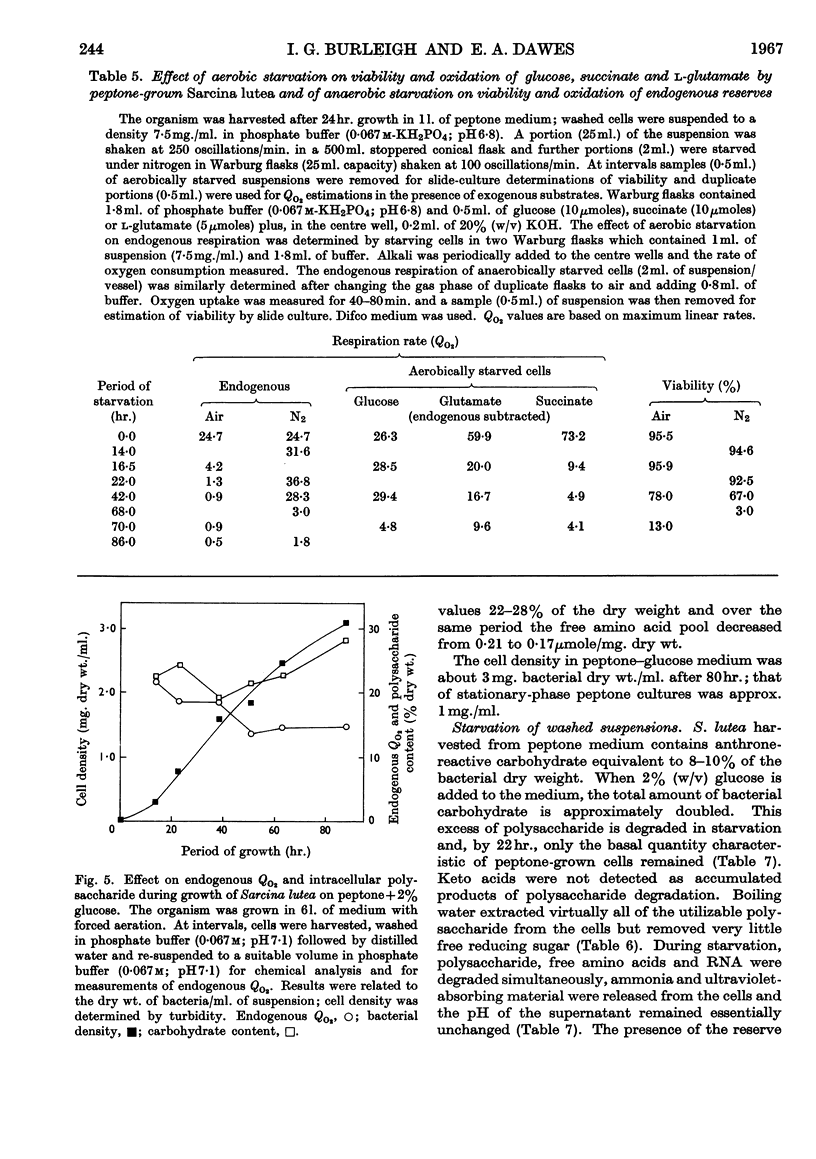
- BINNIE B., DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. IV. Patterns of oxidative assimilation. Biochim Biophys Acta. 1960 May 20;40:237–251. doi: 10.1016/0006-3002(60)91348-2. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. I. Carbohydrate oxidation and terminal respiration. J Bacteriol. 1958 Apr;75(4):390–399. doi: 10.1128/jb.75.4.390-399.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. III. Endogenous metabolism. Biochim Biophys Acta. 1958 Nov;30(2):278–293. doi: 10.1016/0006-3002(58)90052-0. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. The endogenous metabolism of microorganisms. Annu Rev Microbiol. 1962;16:241–264. doi: 10.1146/annurev.mi.16.100162.001325. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Gronlund A. F., Campbell J. J. Enzymatic Degradation of Ribosomes During Endogenous Respiration of Pseudomonas aeruginosa. J Bacteriol. 1965 Jul;90(1):1–7. doi: 10.1128/jb.90.1.1-7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN J. T. Degradation of intracellular nucleic acid and leakage of fragments by Lactobacillus arabinosus. Biochim Biophys Acta. 1958 Sep;29(3):667–668. doi: 10.1016/0006-3002(58)90041-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PAUL J. The chemical determination of deoxyribonucleic acid in tissue cultures. J Biophys Biochem Cytol. 1956 Nov 25;2(6):797–798. doi: 10.1083/jcb.2.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., CRUMPTON J. E., HUNTER J. R. The measurement of bacterial viabilities by slide culture. J Gen Microbiol. 1961 Jan;24:15–24. doi: 10.1099/00221287-24-1-15. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. ACCELERATED DEATH OF AEROBACTER AEROGENES STARVED IN THE PRESENCE OF GROWTH-LIMITING SUBSTRATES. J Gen Microbiol. 1964 Mar;34:459–473. doi: 10.1099/00221287-34-3-459. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. Acceleration of bacterial death by grown substrates. Nature. 1963 Apr 20;198:273–273. doi: 10.1038/198273a0. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SIERRA G., GIBBONS N. E. Role and oxidation pathway of poly-beta-hydroxybutyric acid in Micrococcus halodenitrificans. Can J Microbiol. 1962 Apr;8:255–269. doi: 10.1139/m62-032. [DOI] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. 'SUBSTRATE-ACCELERATED DEATH' OF AEROBACTER AEROGENES. J Gen Microbiol. 1965 May;39:215–228. doi: 10.1099/00221287-39-2-215. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., SHON M. EFFECTS OF THERMAL STRESS ON VIABILITY AND RIBONUCLEIC ACID OF AEROBACTER AEROGENES IN AQUEOUS SUSPENSION. J Gen Microbiol. 1964 Jan;34:99–114. doi: 10.1099/00221287-34-1-99. [DOI] [PubMed] [Google Scholar]
- Stumpf P. K., Green D. E., Smith F. W. Ultrasonic Disintegration as Method of Extracting Bacterial Enzymes. J Bacteriol. 1946 Apr;51(4):487–493. [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. J., FORREST W. W. ANAEROBIC ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1964 Feb;87:256–262. doi: 10.1128/jb.87.2.256-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTER H. Macromolecular ageing in vivo. Nature. 1963 Apr 13;198:189–190. doi: 10.1038/198189b0. [DOI] [PubMed] [Google Scholar]


