Abstract
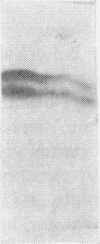
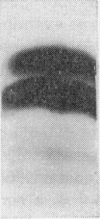
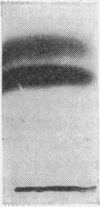
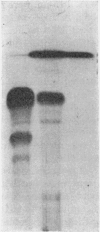
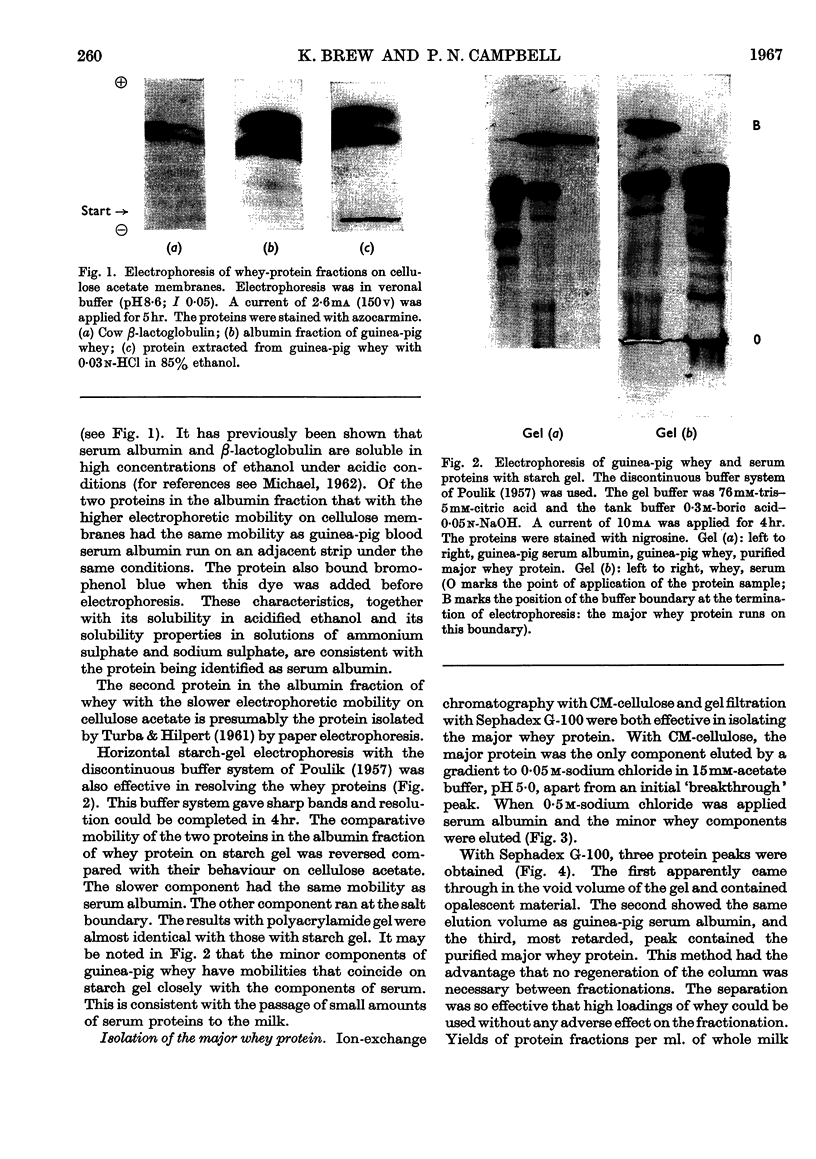
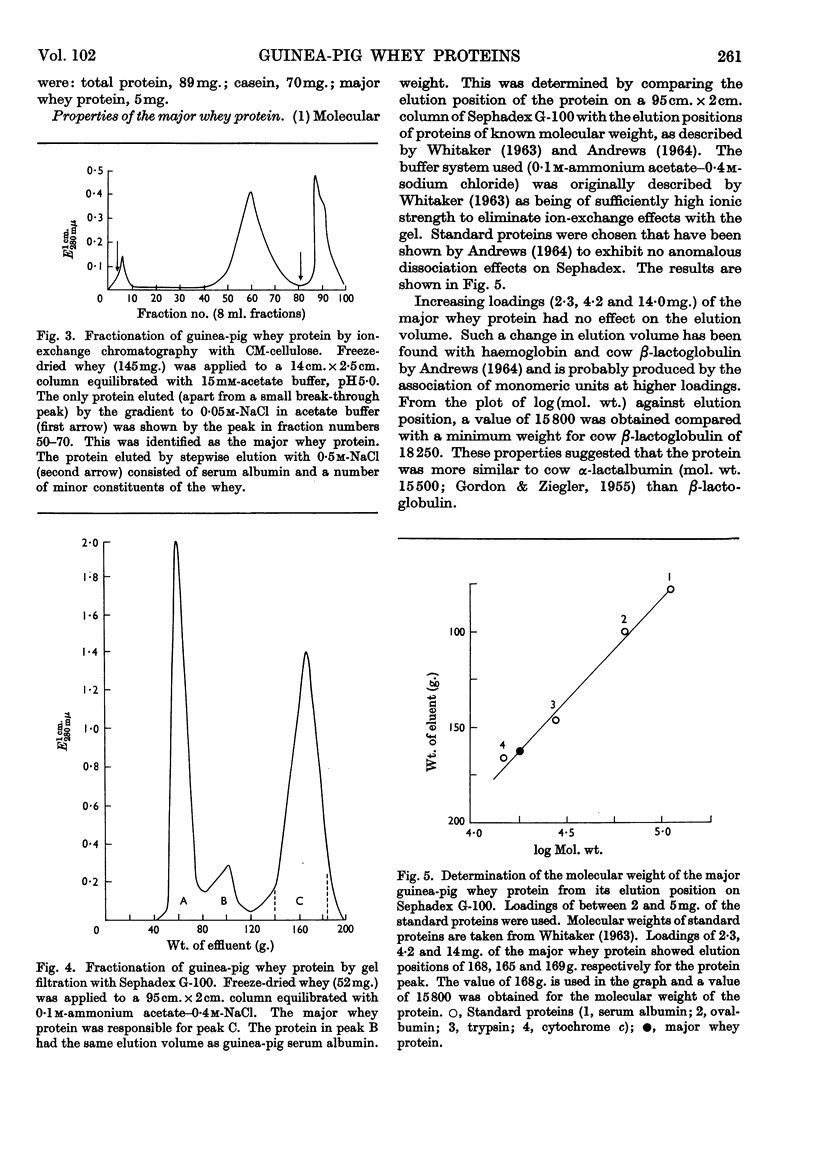
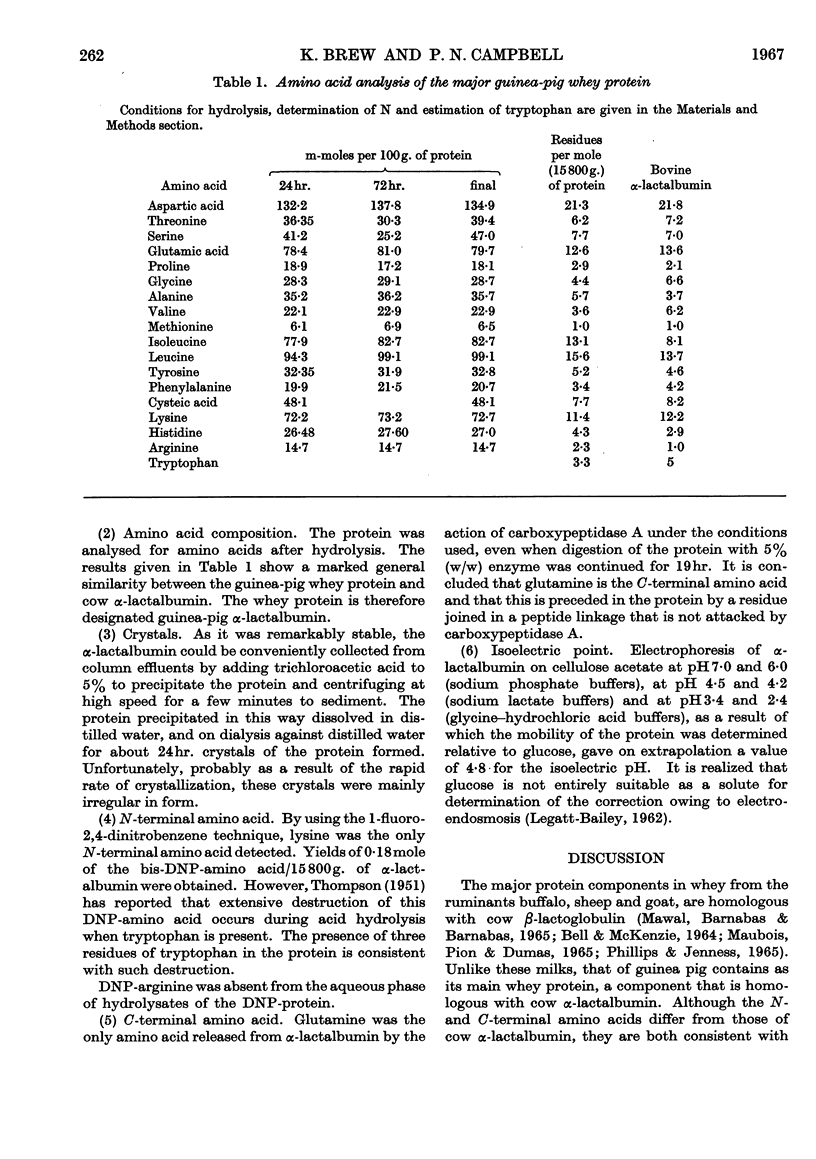
1. The whey proteins of guinea-pig milk were examined by electrophoresis on paper, cellulose acetate, starch gel and polyacrylamide gel. 2. Two major proteins were detected, one of which was identified as blood serum albumin. 3. The major whey protein was isolated by CM-cellulose chromatography and on columns of Sephadex G-100. 4. The amino acid composition of the protein, taken in conjunction with its other properties, indicated that the major whey protein in guinea-pig milk is homologous with cow α-lactalbumin and that β-lactoglobulin is absent from guinea-pig milk. 5. Guinea-pig α-lactalbumin, which was obtained crystalline, had mol.wt. 15800, N-terminal lysine and C-terminal glutamine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHAFFENBURG R., DREWRY J. Improved method for the preparation of crystalline beta-lactoglobulin and alpha-lactalbumin from cow's milk. Biochem J. 1957 Feb;65(2):273–277. doi: 10.1042/bj0650273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL K., MCKENZIE H. A. BETA-LACTOGLOBULINS. Nature. 1964 Dec 26;204:1275–1279. doi: 10.1038/2041275a0. [DOI] [PubMed] [Google Scholar]
- BLACKBURN S., LOWTHER A. G. The separation of N-2:4-dinitrophenly amino-acids on paper chromatograms. Biochem J. 1951 Jan;48(1):126–128. doi: 10.1042/bj0480126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL P. N., GREENGARD O., KERNOT B. A. Studies on the synthesis o serum albumin by the isolated microsome fraction from rat liver. Biochem J. 1960 Jan;74:107–117. doi: 10.1042/bj0740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUMPTON M. J., WILKINSON J. M. AMINO ACID COMPOSITIONS OF HUMAN AND RABBIT GAMMA-GLOBULINS AND OF THE FRAGMENTS PRODUCED BY REDUCTION. Biochem J. 1963 Aug;88:228–234. doi: 10.1042/bj0880228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil R., Ogston A. G. The sedimentation constant, diffusion constant and molecular weight of lactoglobulin. Biochem J. 1949;44(1):33–35. doi: 10.1042/bj0440033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GORDON W. G., ZIEGLER J. Amino acid composition of crystallin alpha-lactalbumin. Arch Biochem Biophys. 1955 Jul;57(1):80–86. doi: 10.1016/0003-9861(55)90179-x. [DOI] [PubMed] [Google Scholar]
- González Cadavid N., Paladini A. C. Amino acid composition of dermal-collagen fractions in rats of different ages. Biochem J. 1964 Aug;92(2):436–439. doi: 10.1042/bj0920436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- JOHANSSON B. Chromatographic separation of lactalbumin from human milk whey on calcium phosphate columns. Nature. 1958 Apr 5;181(4614):996–997. doi: 10.1038/181996b0. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B., MORGAN E. H. THE RELATION BETWEEN THE PROTEINS OF PLASMA AND MILK IN THE RAT. Biochim Biophys Acta. 1965 Apr 12;100:128–135. doi: 10.1016/0304-4165(65)90435-6. [DOI] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- MAWAL R. B., BARNABAS T., BARNABAS J. IDENTITY OF COW BETA-LACTOGLOBULIN 'B' AND BUFFALO BETA-LACTOGLOBULIN. Nature. 1965 Jan 9;205:175–176. doi: 10.1038/205175a0. [DOI] [PubMed] [Google Scholar]
- MICHAEL S. E. The isolation of albumin from blood serum or plasma by means of organic solvents. Biochem J. 1962 Jan;82:212–218. doi: 10.1042/bj0820212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubois J. L., Pion R., Ribadeau-Dumas B. Préparation et étude de la beta-lactoglobuline de brebis cristallisée. Biochim Biophys Acta. 1965 Oct 18;107(3):501–510. [PubMed] [Google Scholar]
- Nirenberg M., Leder P., Bernfield M., Brimacombe R., Trupin J., Rottman F., O'Neal C. RNA codewords and protein synthesis, VII. On the general nature of the RNA code. Proc Natl Acad Sci U S A. 1965 May;53(5):1161–1168. doi: 10.1073/pnas.53.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON A. R. Destruction of dinitrophenyl amino-acids by tryptophane. Nature. 1951 Sep 1;168(4270):390–391. doi: 10.1038/168390a0. [DOI] [PubMed] [Google Scholar]