Abstract
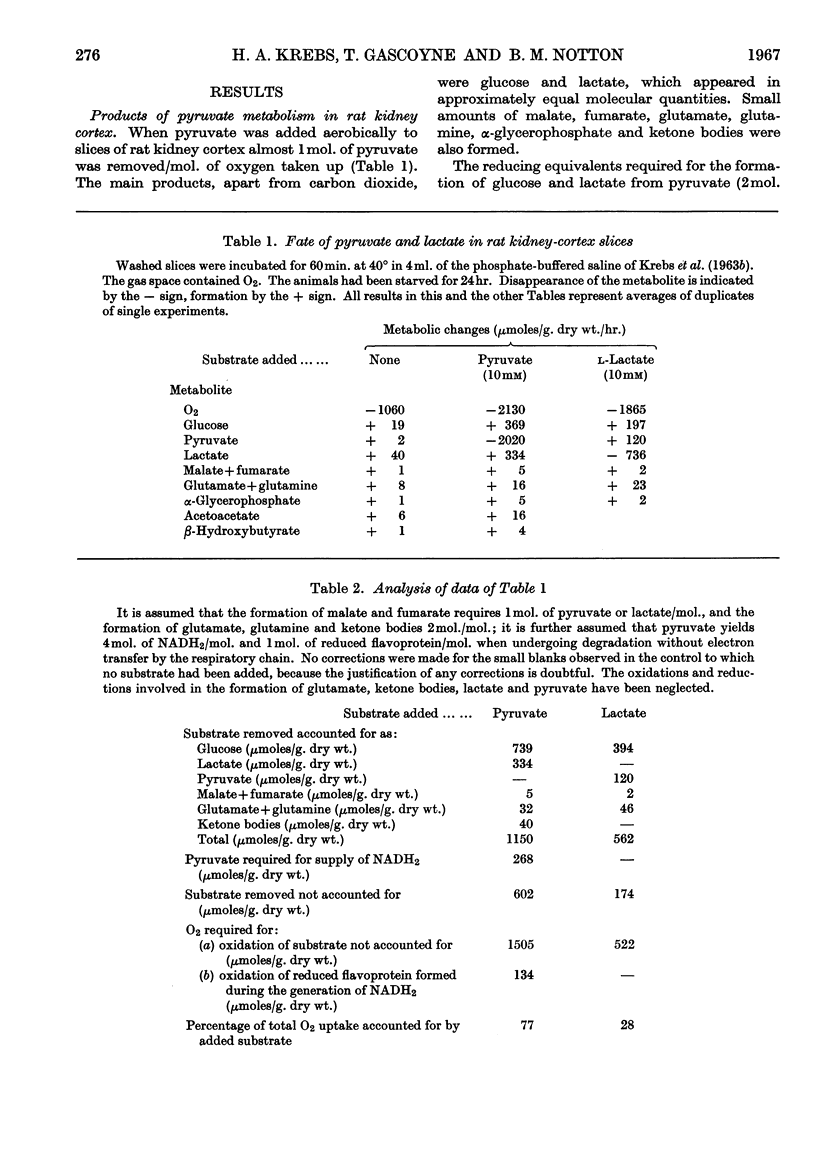
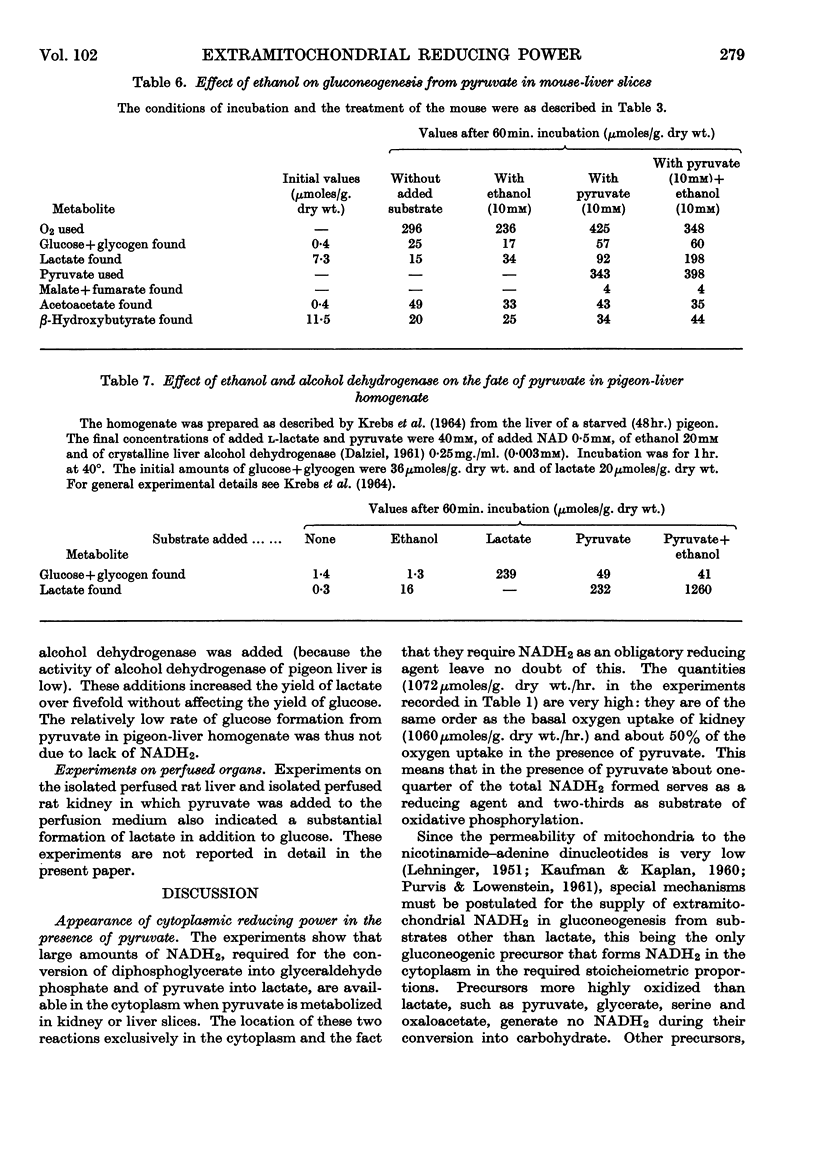
1. Kidney-cortex slices incubated with pyruvate formed glucose and lactate in relatively large and approximately equimolar quantities. The formation of these products involves two exclusively cytoplasmic NADH2-requiring reductions, catalysed by lactate dehydrogenase and triose phosphate dehydrogenase. From the rates of glucose and lactate formation it can be calculated that over 1000μ-moles of NADH2 must have been produced in the cytoplasm/g. dry wt. of tissue/hr. 2. When lactate is a gluconeogenic precursor the required NADH2 is generated in the cytoplasm, but, when a substrate more highly oxidized than glucose, such as pyruvate, is the precursor, there is no direct cytoplasmic source of NADH2. Quantitative data on the fate of pyruvate are in accord with the conclusion that the NADH2 was primarily formed intramitochondrially by the dehydrogenases of cell respiration, with pyruvate as the major substrate. 3. Similar observations and conclusions apply to experiments with mouse-liver slices incubated with pyruvate, serine or aspartate. 4. Addition of ethanol, which increases the formation of NADH2 in the cytoplasm, increased the formation from pyruvate of lactate but not of glucose. 5. In view of the low permeability of mitochondria for NAD and NADH2 it must be postulated that special carrier mechanisms transfer the reducing equivalents of intramitochondrially generated NADH2 to the cytoplasm. Reasons are given in support of the assumption that the malate–oxaloacetate system acts as the carrier. 6. Various aspects of the generation of reducing power and its transfer from mitochondria to cytoplasm are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I. Generation of reducing power in chemosynthesis. II. Energy-linked reduction of pyridine nucleotides in the chemoautotroph, Nitrosomonas europaea. Biochim Biophys Acta. 1966 Feb 14;113(2):216–224. doi: 10.1016/s0926-6593(66)80062-0. [DOI] [PubMed] [Google Scholar]
- BLAYLOCK B. A., NASON A. ELECTRON TRANSPORT SYSTEMS OF THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. I. CYTOCHROME C-CONTAINING IRON OXIDASE. J Biol Chem. 1963 Oct;238:3453–3462. [PubMed] [Google Scholar]
- DALZIEL K. The preparation and properties of crystalline alcohol dehydrogenase from liver. Biochem J. 1961 Aug;80:440–445. doi: 10.1042/bj0800440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSTER L., LEE C. P. BIOLOGICAL OXIDOREDUCTIONS. Annu Rev Biochem. 1964;33:729–790. doi: 10.1146/annurev.bi.33.070164.003501. [DOI] [PubMed] [Google Scholar]
- FITCH W. M., CHAIKOFF I. L. Extent and patterns of adaptation of enzyme activities in livers of normal rats fed diets high in glucose and fructose. J Biol Chem. 1960 Mar;235:554–557. [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBERMAN H. D., D'ADAMO A. F., Jr Coupling of oxidation of substrates to reductive biosyntheses. IV. Studies with 2, 2'-D-fumarate and 2,2'-C14-fumarate. J Biol Chem. 1960 Feb;235:519–522. [PubMed] [Google Scholar]
- Haynes R. C., Jr The fixation of carbon dioxide by rat liver mitochondria and its relation to gluconeogenesis. J Biol Chem. 1965 Oct;240(10):4103–4106. [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN B. T., KAPLAN N. O. Mechanism of depletion of mitochondrial pyridine nucleotides. Biochim Biophys Acta. 1960 Apr 8;39:332–342. doi: 10.1016/0006-3002(60)90171-2. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Considerations concerning the pathways of syntheses in living matter; synthesis of glycogen from non-carbohydrate precursors. Bull Johns Hopkins Hosp. 1954 Jul;95(1):19–33. [PubMed] [Google Scholar]
- KREBS H. A., EGGLESTON L. V., D'ALESSANDRO A. The effect of succinate and amytal on the reduction of acetoacetate in animal tissues. Biochem J. 1961 Jun;79:537–549. doi: 10.1042/bj0790537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R., GASCOYNE T. RENAL GLUCONEOGENESIS. IV. GLUCONEOGENESIS FROM SUBSTRATE COMBINATIONS. Acta Biol Med Ger. 1963;11:607–615. [PubMed] [Google Scholar]
- KULKA R. G., KREBS H. A., EGGLESTON L. V. The reduction of acetoacetate to beta-hydroxybutyrate in animal tissues. Biochem J. 1961 Jan;78:95–106. doi: 10.1042/bj0780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Dierks C., Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. doi: 10.1042/bj0930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Notton B. M., Hems R. Gluconeogenesis in mouse-liver slices. Biochem J. 1966 Dec;101(3):607–617. doi: 10.1042/bj1010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem. 1951 May;190(1):345–359. [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURVIS J. L., LOWENSTEIN J. M. The relation between intra- and extramitochondrial pyridine nucleotides. J Biol Chem. 1961 Oct;236:2794–2803. [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION. J Biol Chem. 1963 Aug;238:2603–2608. [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


