Abstract
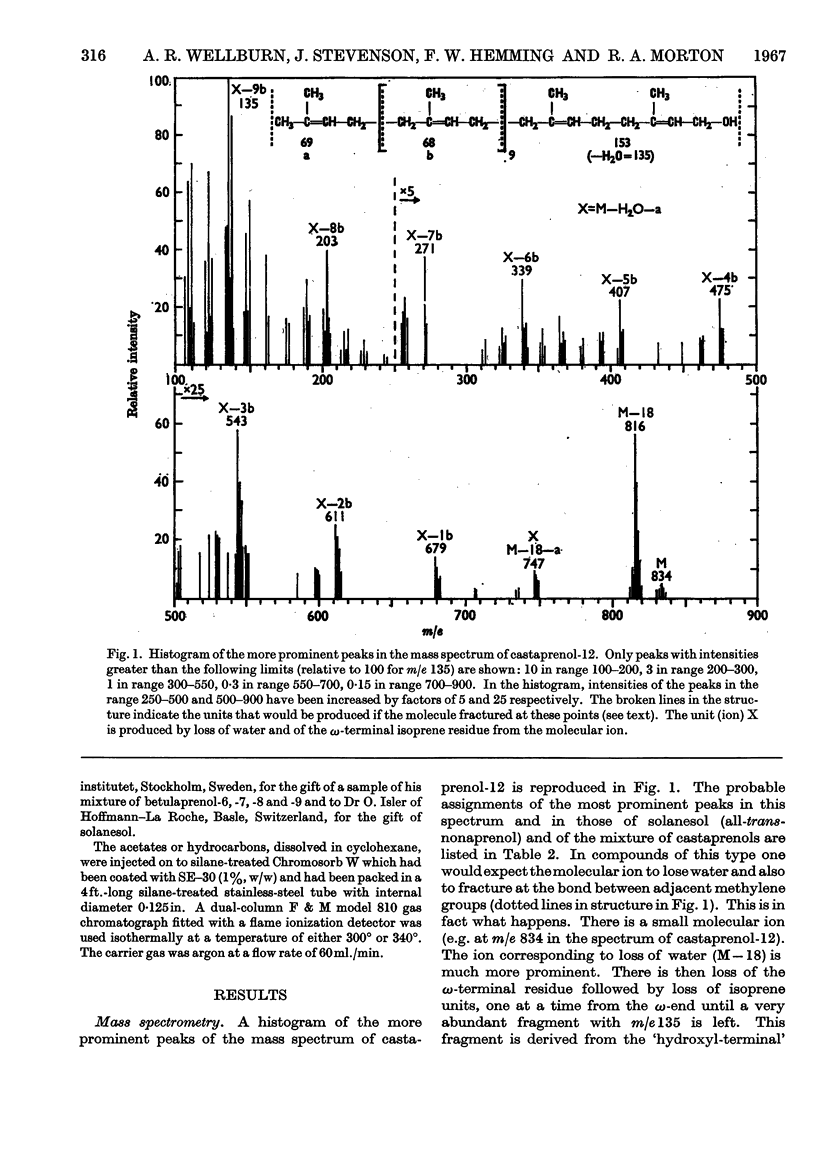
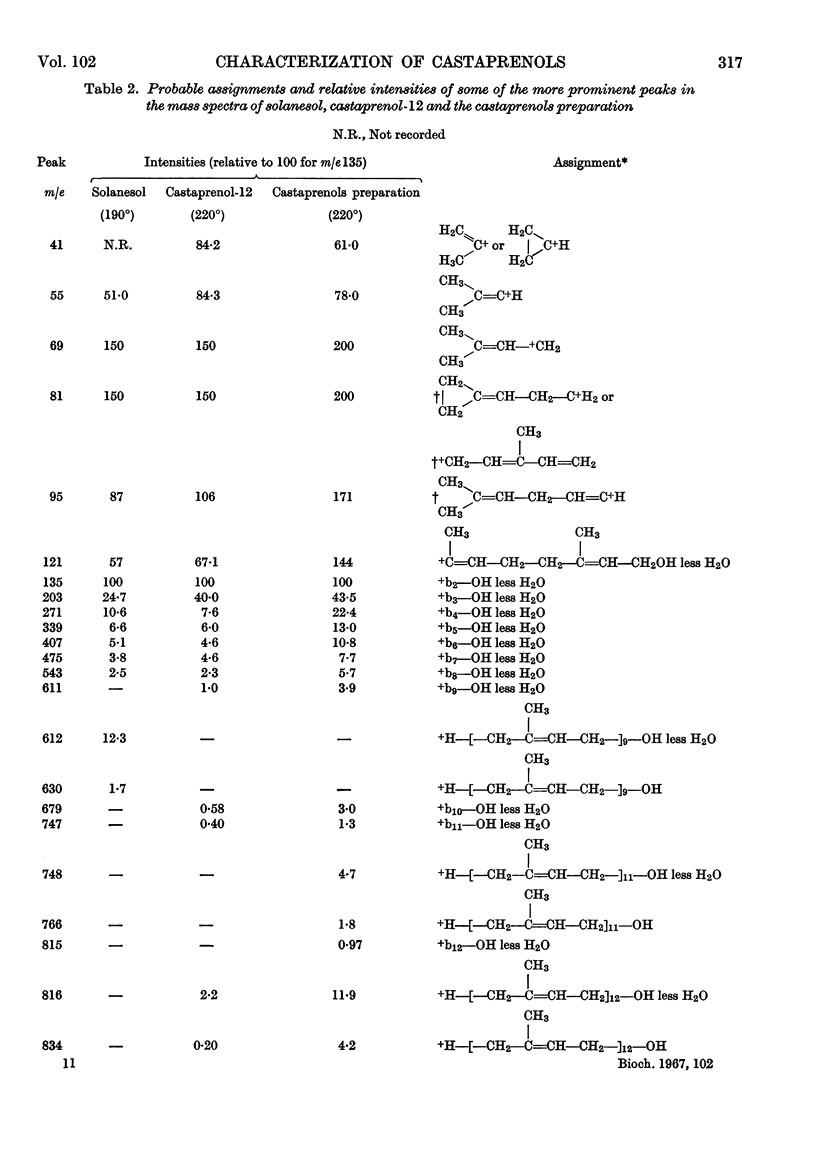
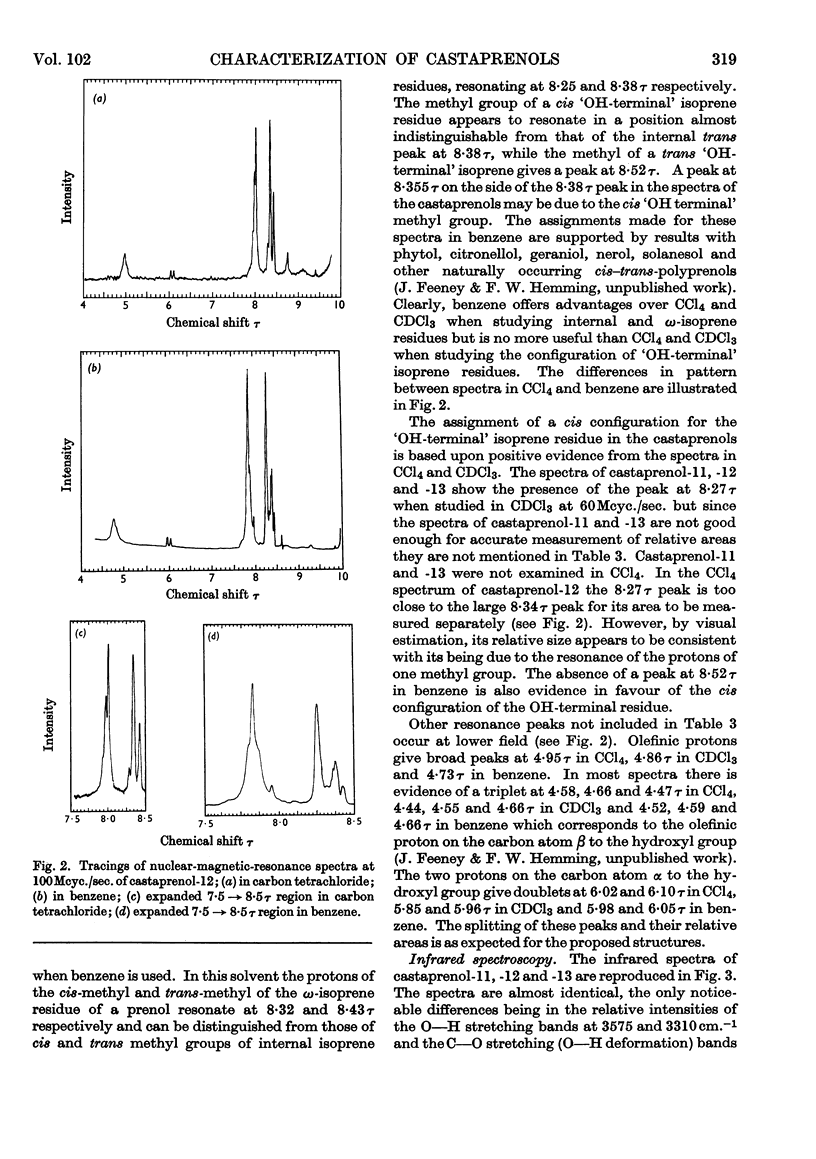
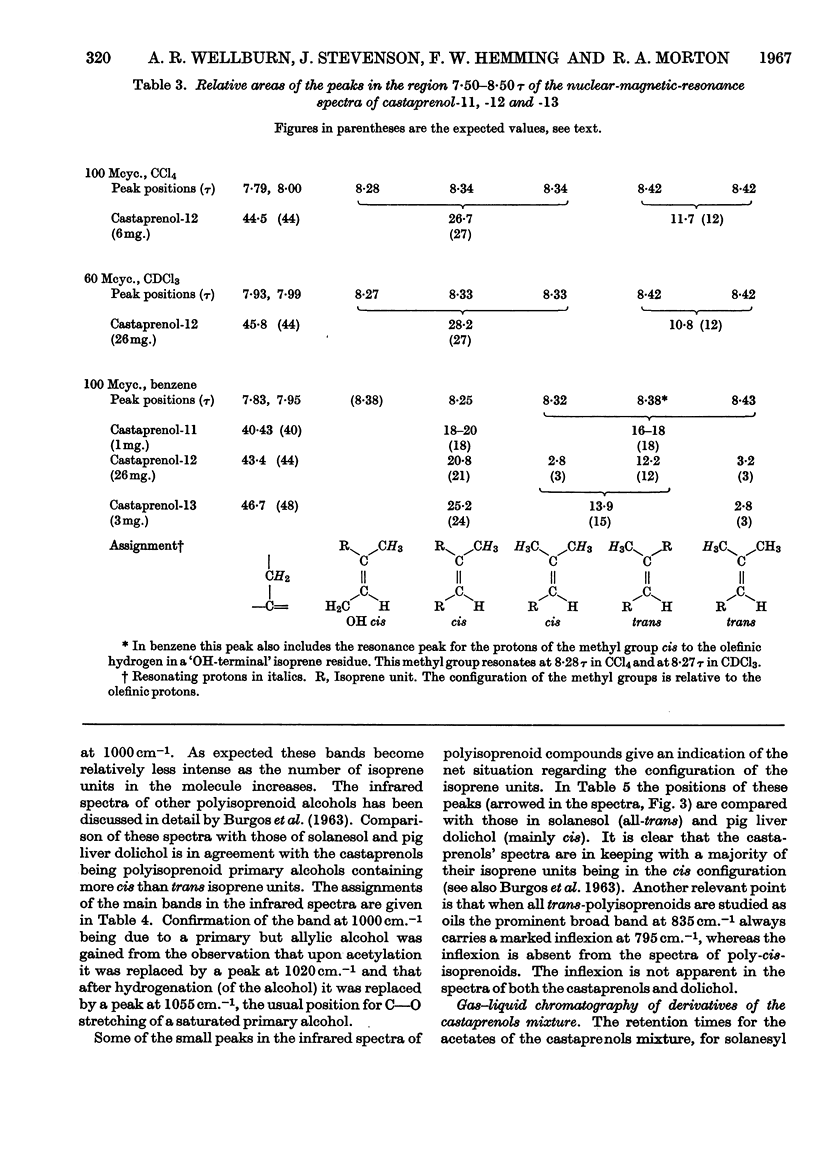
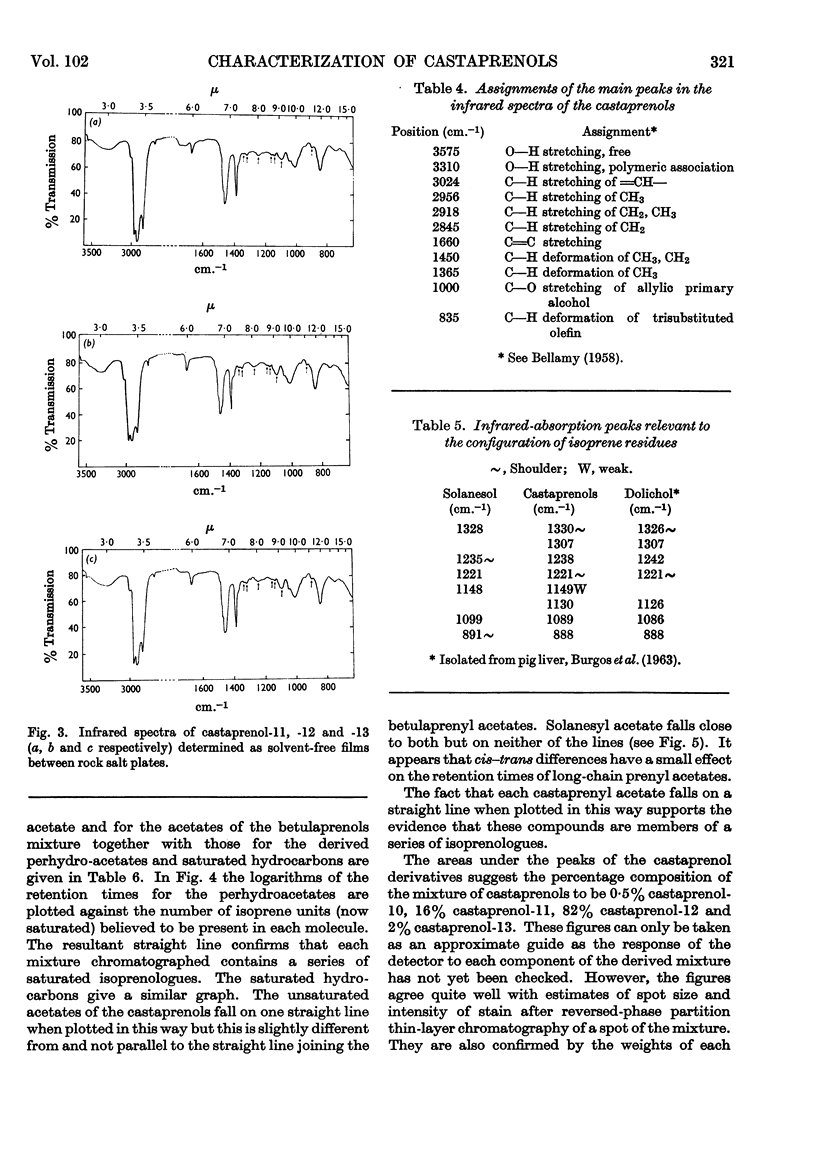
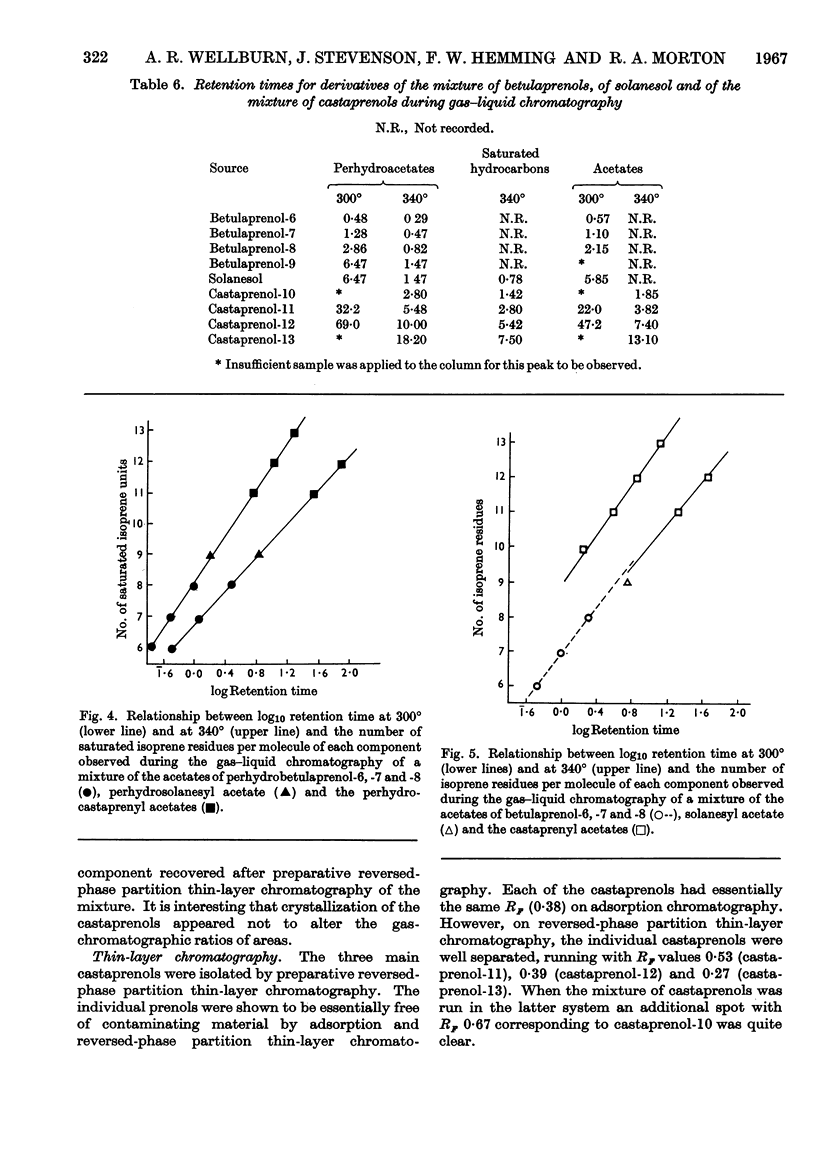
The isolation and purification of a mixture of cis–trans-polyprenols from the leaves of Aesculus hippocastanum (horse chestnut) are described. Results of studies involving mass spectrometry, nuclear magnetic resonance, infrared spectroscopy, micro-hydrogenation and ozonolytic degradation show the mixture to be made up of undecaprenol, dodecaprenol and tridecaprenol with dodecaprenol predominating. Each of the prenols contains three trans internal isoprene residues and a cis `OH-terminal' isoprene residue. They differ from each other only in the number of cis internal isoprene residues. The trivial names castaprenol-11, castaprenol-12 and castaprenol-13 are proposed to describe these compounds. Gas–liquid-chromatographic and reversed-phase partition thin-layer chromatographic evidence suggest the presence in the mixture of small quantities of castaprenol-10 also.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGOS J., HEMMING F. W., PENNOCK J. F., MORTON R. A. DOLICHOL: A NATURALLY-OCCURRING C100 ISOPRENOID ALCOHOL. Biochem J. 1963 Sep;88:470–482. [PMC free article] [PubMed] [Google Scholar]
- Byrne G. A. The separation of 2,4-dinitrophenylhydrazones by thin-layer chromatography. J Chromatogr. 1965 Dec;20(3):528–540. doi: 10.1016/s0021-9673(01)97455-2. [DOI] [PubMed] [Google Scholar]
- Stone K. J., Wellburn A. R., Hemming F. W., Pennock J. F. The characterization of ficaprenol-10, -11 and 12 from the leaves of Ficus elastica (decorative rubber plant). Biochem J. 1967 Jan;102(1):325–330. doi: 10.1042/bj1020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]


