Abstract
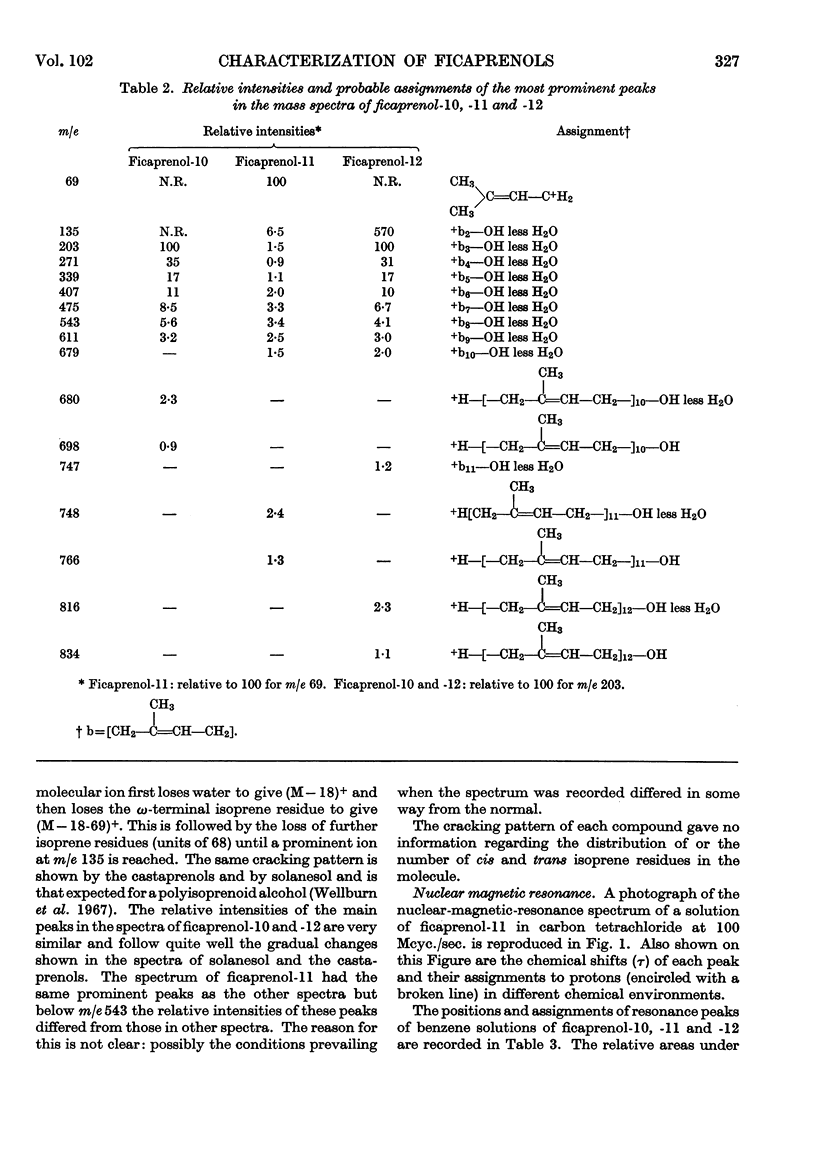
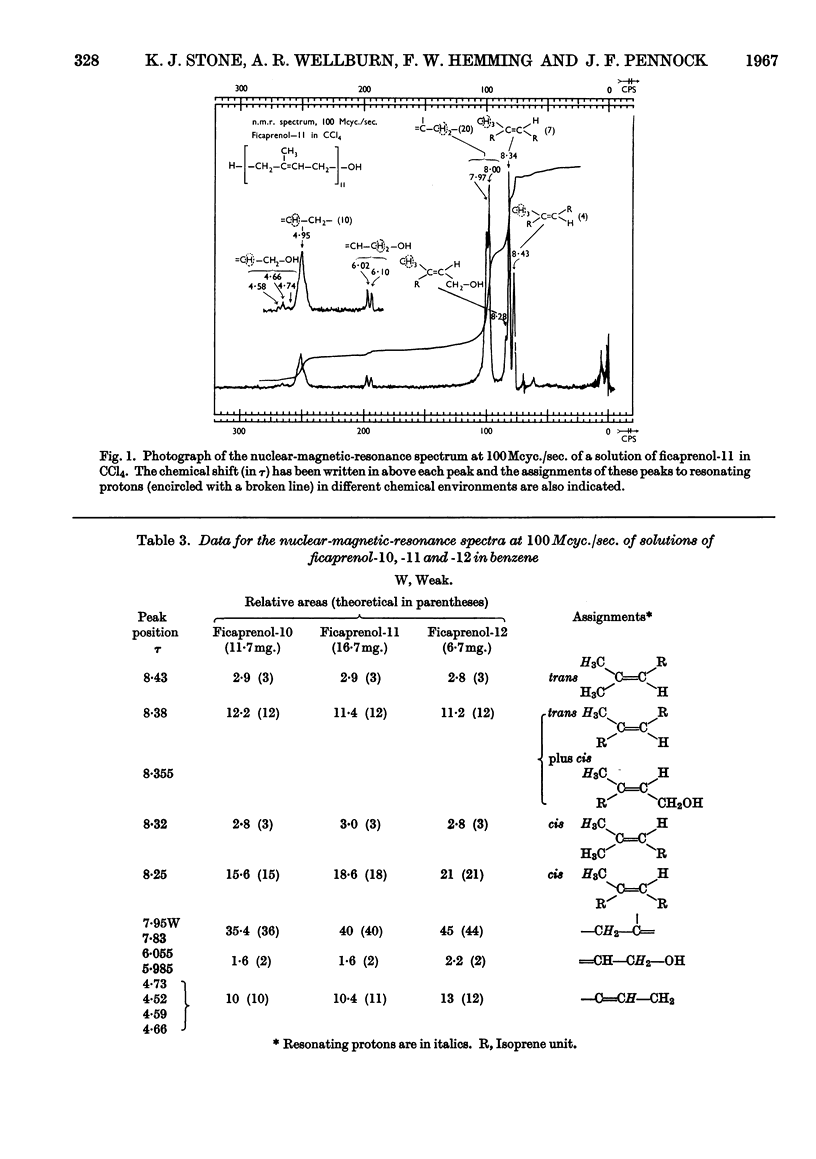
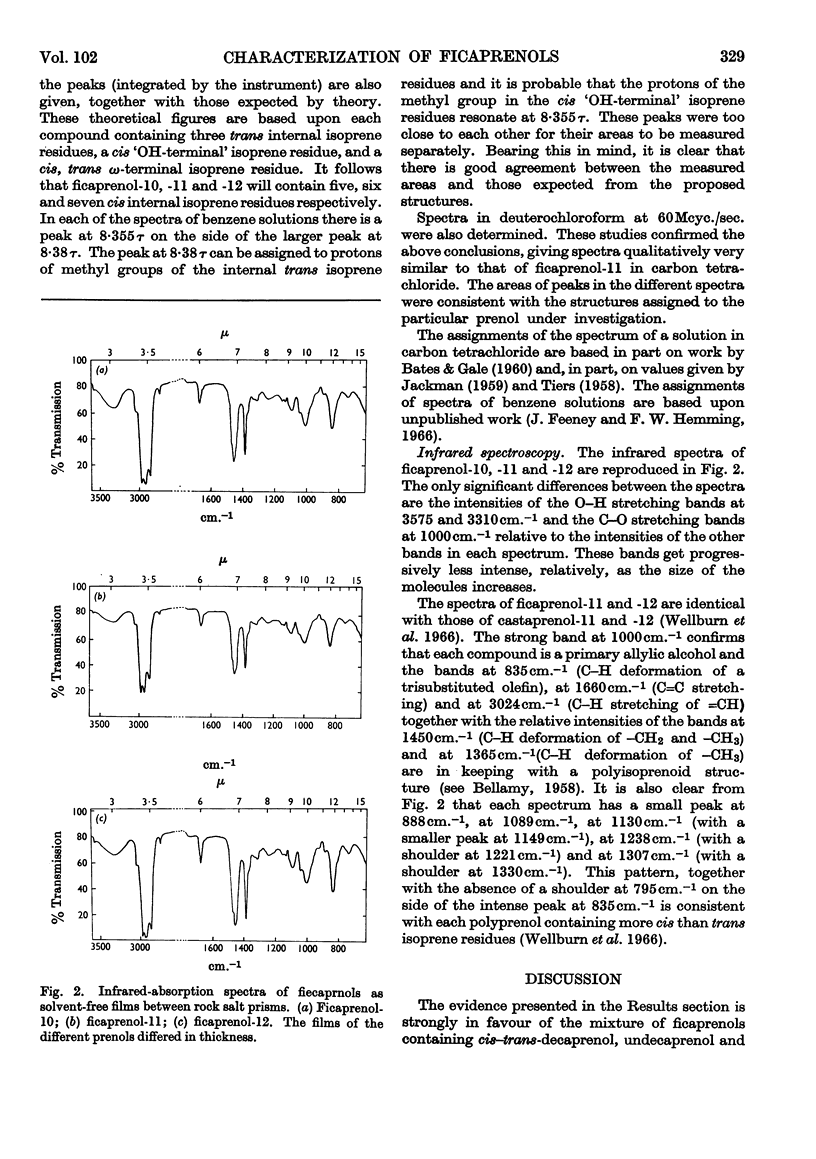
Evidence from mass, nuclear-magnetic-resonance and infrared spectrometry and from gas–liquid and thin-layer chromatography is presented in favour of the presence of cis–trans-decaprenol, -undecaprenol and -dodecaprenol in the mixture of polyprenols (2·6mg./g.) isolated from leaf tissue of Ficus elastica. The trivial names ficaprenol-10, -11 and -12 are proposed. Nuclear-magnetic-resonance studies showed that each of these prenols contains three trans internal isoprene residues and a cis `OH-terminal' isoprene residue. Ficaprenol-11 is the major component of the mixture. Chromatographic evidence suggests the presence also of small amounts of ficaprenol-9 and -13. The precise position of the three trans internal isoprene residues was not determined but it is suggested that these are adjacent to the ω-terminal isoprene residue and that the ficaprenols are formed from all-trans-geranylgeranyl pyrophosphate. It is also suggested that ficaprenol-10, -11, -12 and -13 are probably the same compounds as castaprenol-10, -11, -12 and -13.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wellburn A. R., Stevenson J., Hemming F. W., Morton R. A. The characterization and properties of castaprenol-11, -12 and -13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem J. 1967 Jan;102(1):313–324. doi: 10.1042/bj1020313. [DOI] [PMC free article] [PubMed] [Google Scholar]


