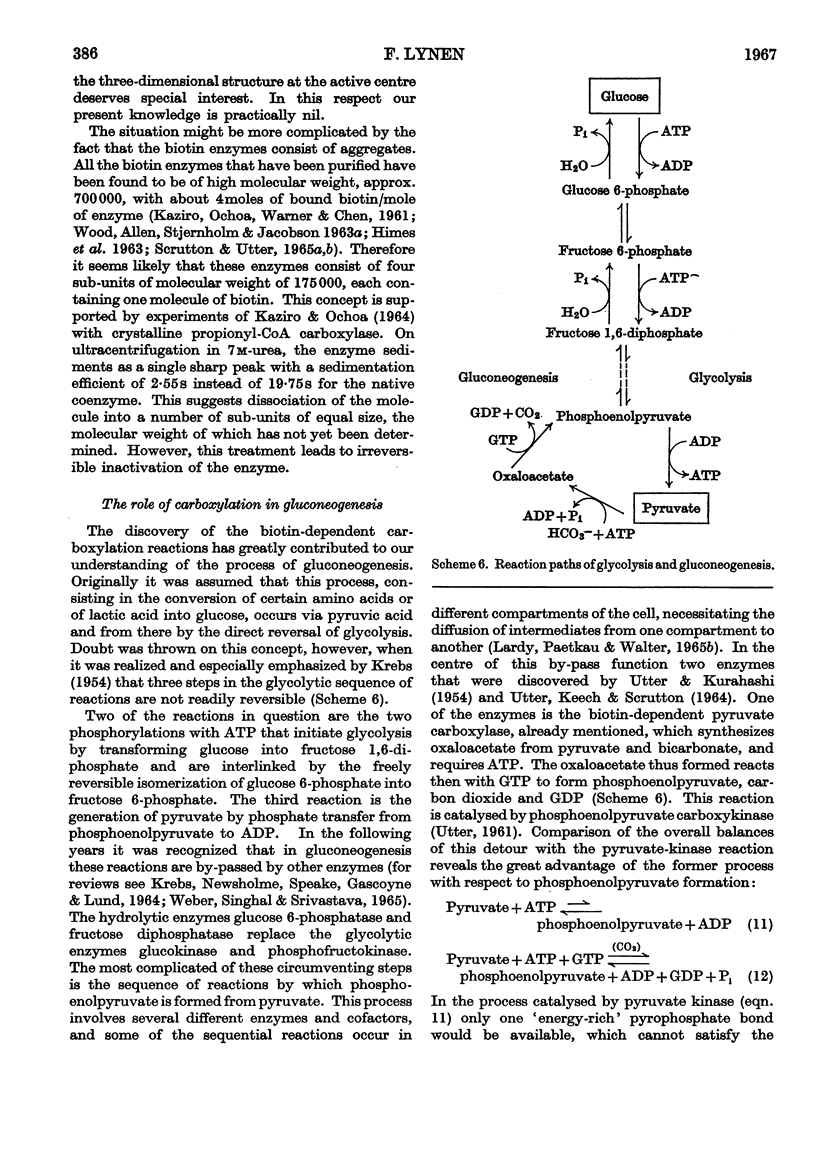
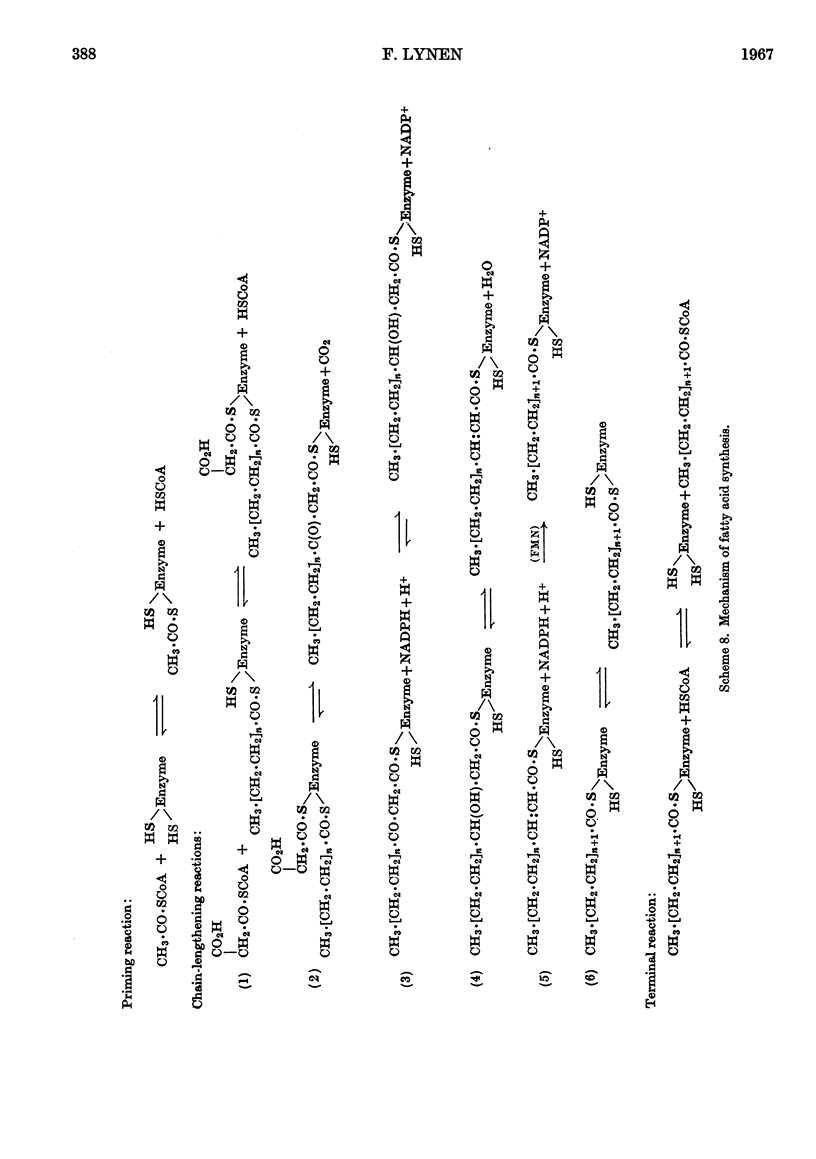
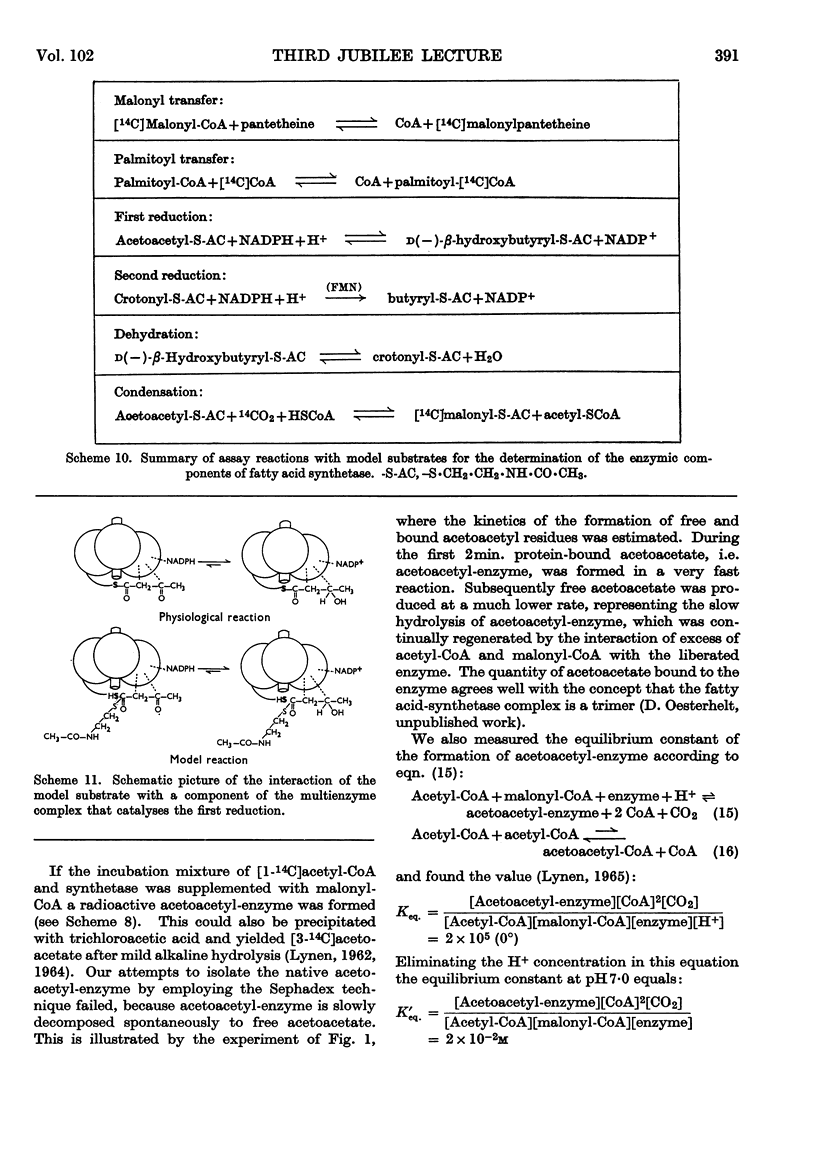
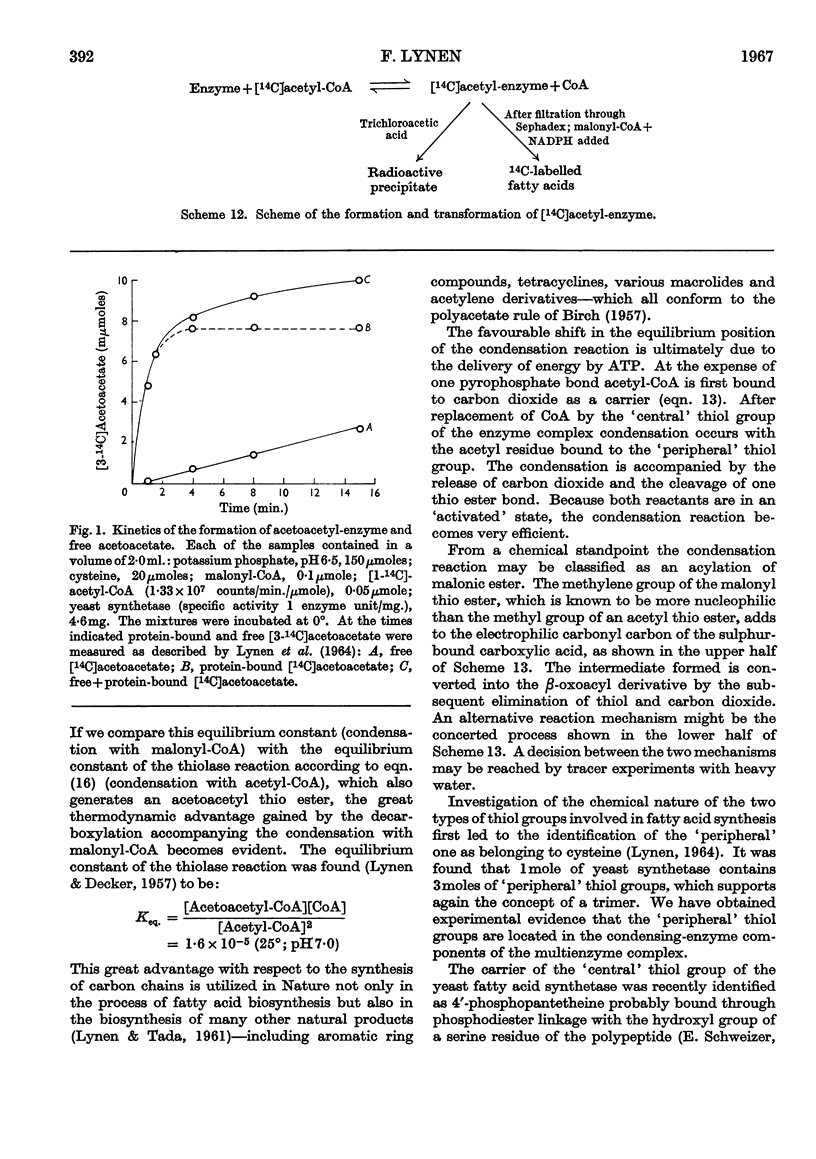
Full text
PDF






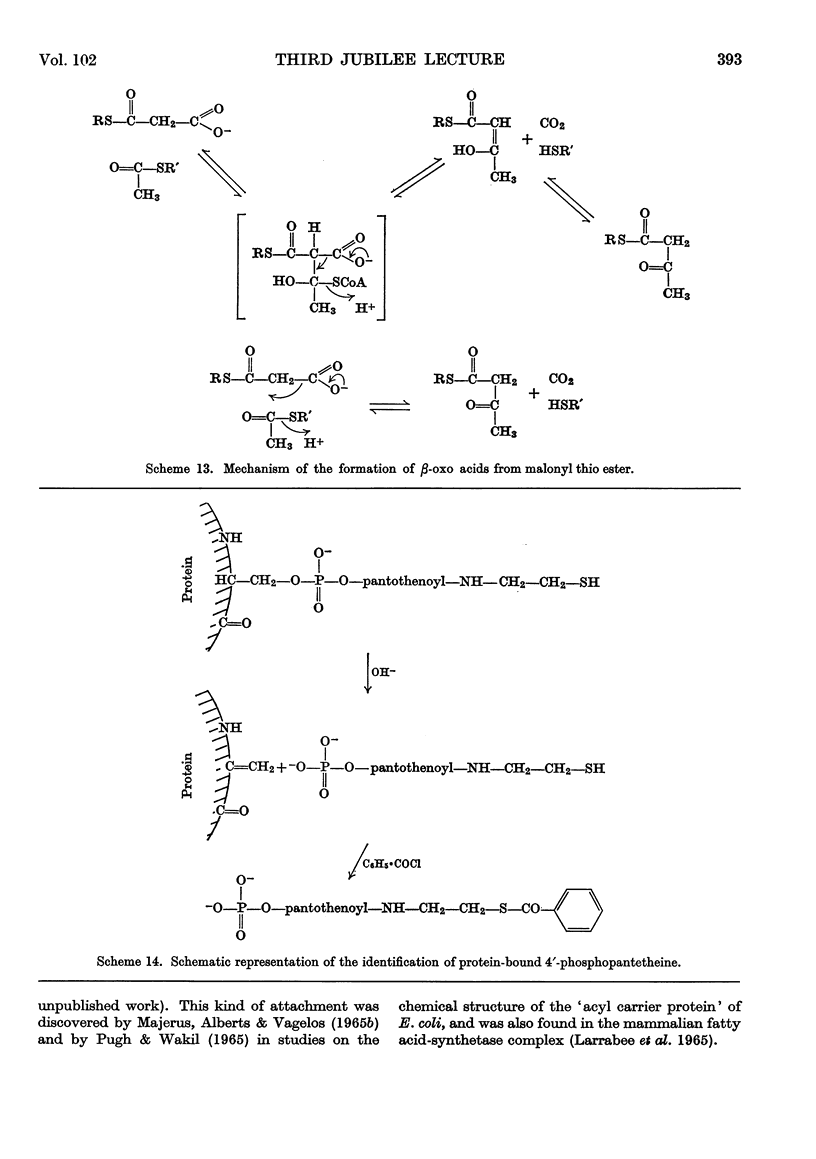
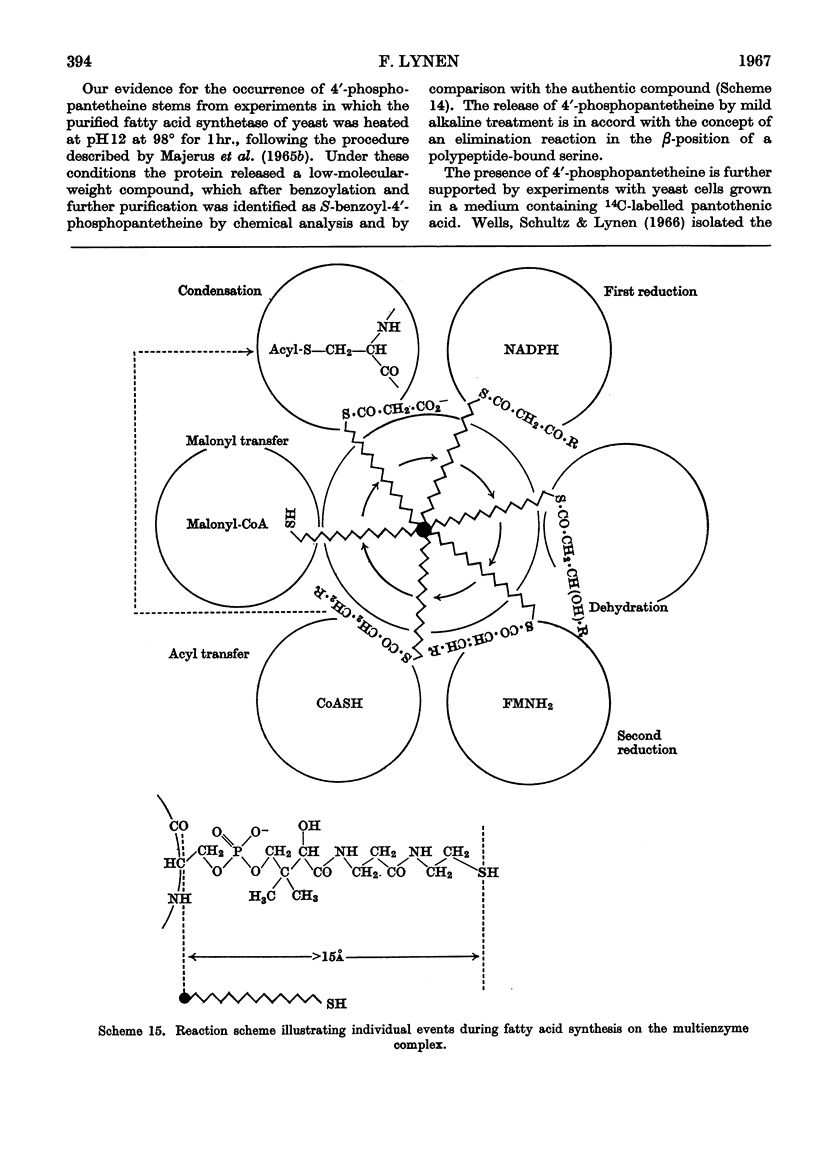
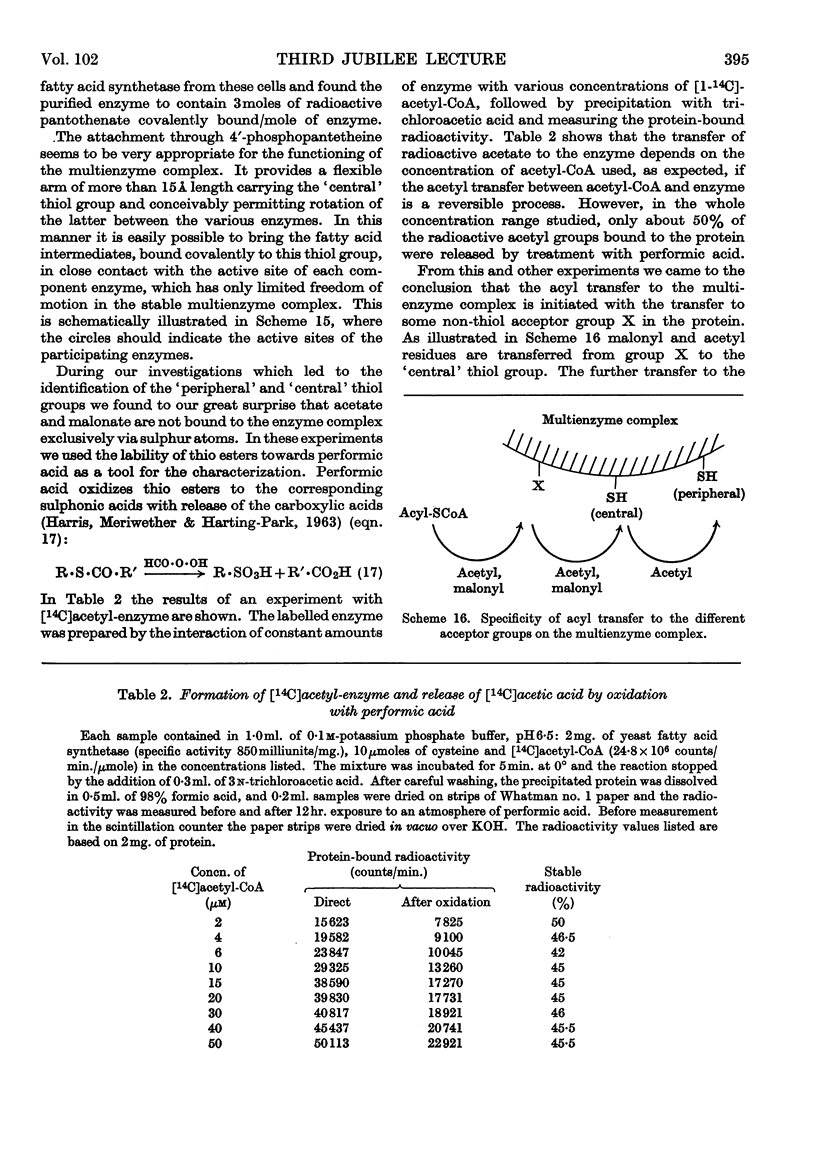
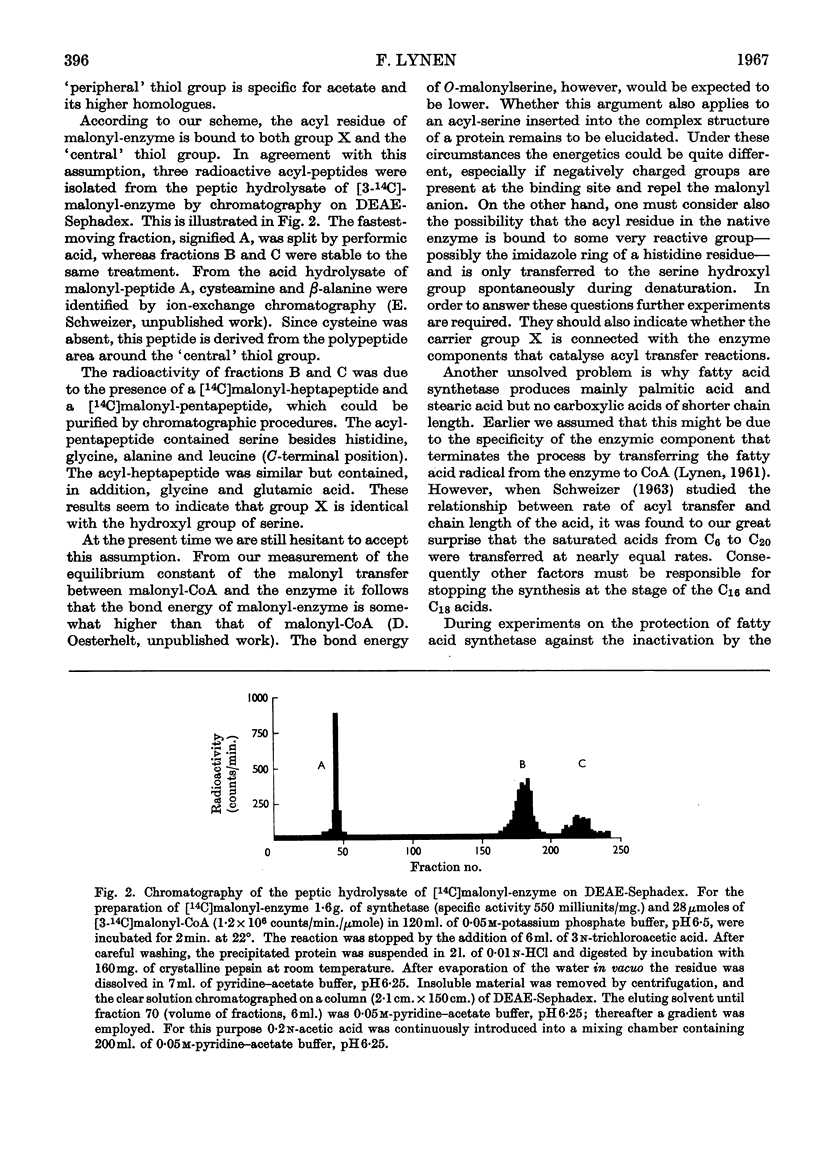



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTS A. W., MAJERUS P. W., TALAMO B., VAGELOS P. R. ACYL-CARRIER PROTEIN. II. INTERMEDIARY REACTIONS OF FATTY ACID SYNTHESIS. Biochemistry. 1964 Oct;3:1563–1571. doi: 10.1021/bi00898a030. [DOI] [PubMed] [Google Scholar]
- BACHHAWAT B. K., ROBINSON W. G., COON M. J. The enzymatic cleavage of beta-hydroxy-beta-methylglutaryl coenzyme A to acetoacetate and acetyl coenzyme A. J Biol Chem. 1955 Oct;216(2):727–736. [PubMed] [Google Scholar]
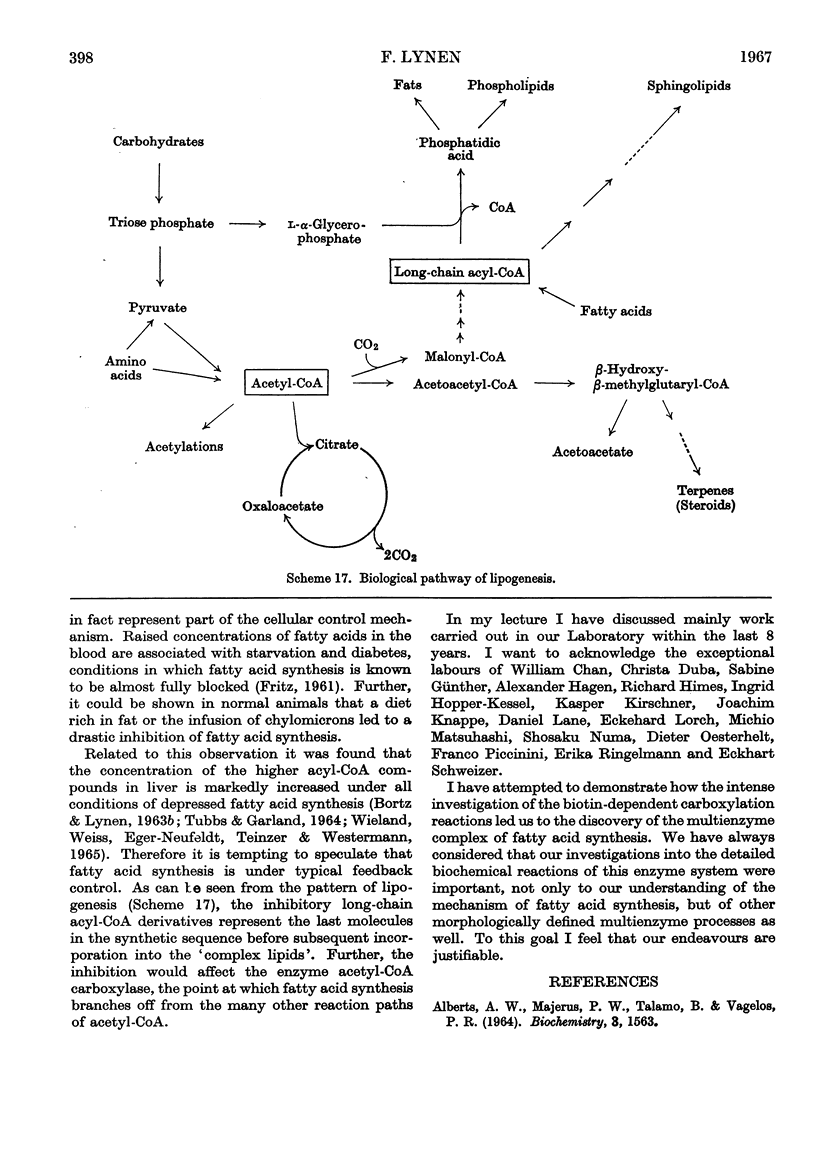
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- GRAVES J. L., PENNINGTON R. J., UTTER M. F., VENNESLAND B. The mechanism of the reversible carboxylation of phosphoenolpyruvate. J Biol Chem. 1956 Nov;223(1):551–557. [PubMed] [Google Scholar]
- György P., Melville D. B., Burk D., DU Vigneaud V. THE POSSIBLE IDENTITY OF VITAMIN H WITH BIOTIN AND COENZYME R. Science. 1940 Mar 8;91(2358):243–245. doi: 10.1126/science.91.2358.243. [DOI] [PubMed] [Google Scholar]
- HARRIS I., MERIWETHER B. P., PARK J. H. Chemical nature of the catalytic sites in glyceraldehyde-3-phosphate dehydrogenase. Nature. 1963 Apr 13;198:154–157. doi: 10.1038/198154a0. [DOI] [PubMed] [Google Scholar]
- HENNING H. V., SEIFFERT I., SEUBERT W. CORTISOL INDUZIERTER ANSTIEG DER PYRUVATCARBOXYLASEAKTIVITAET IN DER RATTENLEBER. Biochim Biophys Acta. 1963 Oct 1;77:345–348. doi: 10.1016/0006-3002(63)90509-2. [DOI] [PubMed] [Google Scholar]
- Hsu R. Y., Wasson G., Porter J. W. The purification and properties of the fatty acid synthetase of pigeon liver. J Biol Chem. 1965 Oct;240(10):3736–3746. [PubMed] [Google Scholar]
- KAZIRO Y., HASS L. F., BOYER P. D., OCHOA S. Mechanism of the propionyl carboxylase reaction. II. Isotopic exchange and tracer experiments. J Biol Chem. 1962 May;237:1460–1468. [PubMed] [Google Scholar]
- KAZIRO Y., OCHOA S. THE METABOLISM OF PROPIONIC ACID. Adv Enzymol Relat Areas Mol Biol. 1964;26:283–378. doi: 10.1002/9780470122716.ch7. [DOI] [PubMed] [Google Scholar]
- KAZIRO Y., OCHOA S., WARNER R. C., CHEN J. Y. Metabolism of propionic acid in animal tissues. VIII. Crystalline propionyl carboxylase. J Biol Chem. 1961 Jul;236:1917–1923. [PubMed] [Google Scholar]
- KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. II. PROPERTIES. J Biol Chem. 1963 Aug;238:2609–2614. [PubMed] [Google Scholar]
- KNAPPE J., RINGELMANN E., LYNEN F. [On the biochemical function of biotin. III. The chemical structure of enzymatically formed carboxy-biotins]. Biochem Z. 1961;335:168–176. [PubMed] [Google Scholar]
- KNAPPE J., WENGER G., WIEGAND U. [On the structurre of carboxylated beta-methylcrotonyl carboxylase (CO2-biotin enzyme)]. Biochem Z. 1963;337:232–246. [PubMed] [Google Scholar]
- KURAHASHI K., PENNINGTON R. J., UTTER M. F. Nucleotide specificity of oxalacetic carboxylase. J Biol Chem. 1957 Jun;226(2):1059–1075. [PubMed] [Google Scholar]
- Krebs H. A., Newsholme E. A., Speake R., Gascoyne T., Lund P. Some factors regulating the rate of gluconeogenesis in animal tissues. Adv Enzyme Regul. 1964;2:71–81. doi: 10.1016/s0065-2571(64)80006-6. [DOI] [PubMed] [Google Scholar]
- LANE M. D., LYNEN F. The biochemical function of biotin, VI. Chemical structure of the carboxylated active site of propionyl carboxylase. Proc Natl Acad Sci U S A. 1963 Mar 15;49:379–385. doi: 10.1073/pnas.49.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., PEANASKY R. Metabolic functions of biotin. Physiol Rev. 1953 Oct;33(4):560–565. doi: 10.1152/physrev.1953.33.4.560. [DOI] [PubMed] [Google Scholar]
- LYNEN F. Biosynthesis of saturated fatty acids. Fed Proc. 1961 Dec;20:941–951. [PubMed] [Google Scholar]
- LYNEN F., DECKER K. Das Coenzym A und seine biologischen Funktionen. Ergeb Physiol. 1957;49:327–424. [PubMed] [Google Scholar]
- LYNEN F., KNAPPE J., LORCH E., JUETTING G., RINGELMANN E., LACHANCE J. P. [On the biochemical function of biotin. II. Purification and mode of action of beta-methyl-crotonyl-carboxylase]. Biochem Z. 1961;335:123–167. [PubMed] [Google Scholar]
- Larrabee A. R., McDaniel E. G., Bakerman H. A., Vagelos P. R. Acyl carrier protein. V. Identification of 4'-phosphopantetheine bound to a mammalian fatty acid synthetase preparation. Proc Natl Acad Sci U S A. 1965 Jul;54(1):267–273. doi: 10.1073/pnas.54.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJERUS P. W., ALBERTS A. W., VAGELOS P. R. THE ACYL CARRIER PROTEIN OF FATTY ACID SYNTHESIS: PURIFICATION, PHYSICAL PROPERTIES, AND SUBSTRATE BINDING SITE. Proc Natl Acad Sci U S A. 1964 Jun;51:1231–1238. doi: 10.1073/pnas.51.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D. B., VAGELOS P. R. The mechanism of tricarboxylic acid cycle regulation of fatty acid synthesis. J Biol Chem. 1962 Jun;237:1787–1792. [PubMed] [Google Scholar]
- MATSUHASHI M., MATSUHASHI S., LYNEN F. ZUR BIOSYNTHESE DER FETTSAEUREN. V. DIE ACETYL-COA CARBOXYLASE AUS RATTENLEBER UND IHRE AKTIVIERUNG DURCH CITRONENSAEURE. Biochem Z. 1964 Aug 11;340:263–289. [PubMed] [Google Scholar]
- Majerus P. W., Alberts A. W., Vagelos P. R. Acyl carrier protein. VII. The primary structure of the substrate-binding site. J Biol Chem. 1965 Dec;240(12):4723–4726. [PubMed] [Google Scholar]
- McQUATE J. T., UTTER M. F. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem. 1959 Aug;234(8):2151–2157. [PubMed] [Google Scholar]
- NUMA S., RINGELMANN E., LYNEN F. ZUR BIOCHEMISCHEN FUNKTION DES BIOTINS. 8. DIE BINDUNG DER KOHLENSAEURE IN DER CARBOXYLIERTEN ACETYL-COA-CARBOXYLASE. Biochem Z. 1964 Jul 29;340:228–242. [PubMed] [Google Scholar]
- Numa S., Ringelmann E., Lynen F. Zur Hemmung der Acetyl-CoA-Carboxylase durch Fettsäure-Coenzym A-Verbindungen. Biochem Z. 1965 Dec 1;343(3):243–257. [PubMed] [Google Scholar]
- Numa S., Ringelmann E. Zur Aufhebung der Citrat-Aktivierung der Acetyl-CoA-Carboxylase durch Kälte. Biochem Z. 1965 Dec 1;343(3):258–268. [PubMed] [Google Scholar]
- OVERATH P., STUMPF P. K. FAT METABOLISM IN HIGHER PLANTS. 23. PROPERTIES OF A SOLUBLE FATTY ACID SYNTHETASE FROM AVOCADO MESOCARP. J Biol Chem. 1964 Dec;239:4103–4110. [PubMed] [Google Scholar]
- Pugh E. L., Wakil S. J. Studies on the mechanism of fatty acid synthesis. XIV. The prosthetic group of acyl carrier protein and the mode of its attachment to the protein. J Biol Chem. 1965 Dec;240(12):4727–4733. [PubMed] [Google Scholar]
- Rétey J., Lynen F. Zur biochemischen Funktion des Biotins. IX. Der sterische Verlauf der Carboxylierung von Propionyl-CoA. Biochem Z. 1965 Aug 6;342(3):256–271. [PubMed] [Google Scholar]
- SAUER F., PUGH E. L., WAKIL S. J., DELANEY R., HILL R. L. 2-MERCAPTOETHYLAMINE AND BETA-ALANINE AS COMPONENTS OF ACYL CARRIER PROTEIN. Proc Natl Acad Sci U S A. 1964 Dec;52:1360–1366. doi: 10.1073/pnas.52.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCRUTTON M. C., KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. IV. PARTIAL REACTIONS AND THE LOCUS OF ACTIVATION BY ACETYL COENZYME A. J Biol Chem. 1965 Feb;240:574–581. [PubMed] [Google Scholar]
- SCRUTTON M. C., UTTER M. F. PYRUVATE CARBOXYLASE. 3. SOME PHYSICAL AND CHEMICAL PROPERTIES OF THE HIGHLY PURIFIED ENZYME. J Biol Chem. 1965 Jan;240:1–9. [PubMed] [Google Scholar]
- SEUBERT W., FASS E., REMBERGER U. UNTERSUCHUNGEN UEBER DEN BAKTERIELLEN ABBAU VON ISOPRENOIDEN. III. REINIGUNG UND EIGENSCHAFTEN DER GERANYLCARBOXYLASE. Biochem Z. 1963;338:265–275. [PubMed] [Google Scholar]
- Scrutton M. C., Utter M. F. Pyruvate carboxylase. V. Interaction of the enzyme with adenosine triphosphate. J Biol Chem. 1965 Oct;240(10):3714–3723. [PubMed] [Google Scholar]
- Shrago E., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. II. Conversion of precursors to phosphoenolpyruvate in liver cytosol. J Biol Chem. 1966 Feb 10;241(3):663–668. [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Purification of oxalacetic carboxylase from chicken liver. J Biol Chem. 1954 Apr;207(2):787–802. [PubMed] [Google Scholar]
- Utter M. F., Keech D. B., Scrutton M. C. A possible role for acetyl CoA in the control of gluconeogenesis. Adv Enzyme Regul. 1964;2:49–68. doi: 10.1016/s0065-2571(64)80005-4. [DOI] [PubMed] [Google Scholar]
- WAITE M., WAKIL S. J. Studies on the mechanism of action of acetyl coenzyme A carboxylase. I. Effect of isocitrate on the transcarboxylation step of acetyl coenzyme A carboxylase. J Biol Chem. 1963 Jan;238:77–80. [PubMed] [Google Scholar]
- WAKIL S. J., PUGH E. L., SAUER F. THE MECHANISM OF FATTY ACID SYNTHESIS. Proc Natl Acad Sci U S A. 1964 Jul;52:106–114. doi: 10.1073/pnas.52.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL S. J., TITCHENER E. B., GIBSON D. M. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim Biophys Acta. 1958 Jul;29(1):225–226. doi: 10.1016/0006-3002(58)90177-x. [DOI] [PubMed] [Google Scholar]
- WESSMAN G. E., WERKMAN C. H. Biotin in the assimilation of heavy carbon in Oxalacetate. Arch Biochem. 1950 Apr;26(2):214–218. [PubMed] [Google Scholar]
- WOOD H. G., ALLEN S. H., STJERNHOLM R., JACOBSON B. Transcarboxylase. III. Purification and properties of methylmalonyl-oxaloacetic transcarboxylase containing tritiated biotin. J Biol Chem. 1963 Feb;238:547–556. [PubMed] [Google Scholar]
- WOOD H. G., LOCHMULLER H., RIEPERTINGER C., LYNEN F. Transcarboxylase. IV. Function of biotin and the structure and properties of the carboxylated enzyme. Biochem Z. 1963;337:247–266. [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Stamm N. B., Fisher E. A., Mentendiek M. A. Regulation of enzymes involved in gluconeogenesis. Adv Enzyme Regul. 1964;2:1–38. doi: 10.1016/s0065-2571(64)80003-0. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Schultz J., Lynen F. Incorporation of C14-pantothenate into the fatty acid synthetase complex of growing baker's yeast. Proc Natl Acad Sci U S A. 1966 Aug;56(2):633–639. doi: 10.1073/pnas.56.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O., Weiss L., Eger-Neufeldt I., Teinzer A., Westermann B. Coenzym A-Thioester höherer Fettsäuren als mögliche Vermittler enzymatischer Regulationen im Tierkörper. Klin Wochenschr. 1965 Jun 15;43(12):645–654. doi: 10.1007/BF01728702. [DOI] [PubMed] [Google Scholar]