Abstract
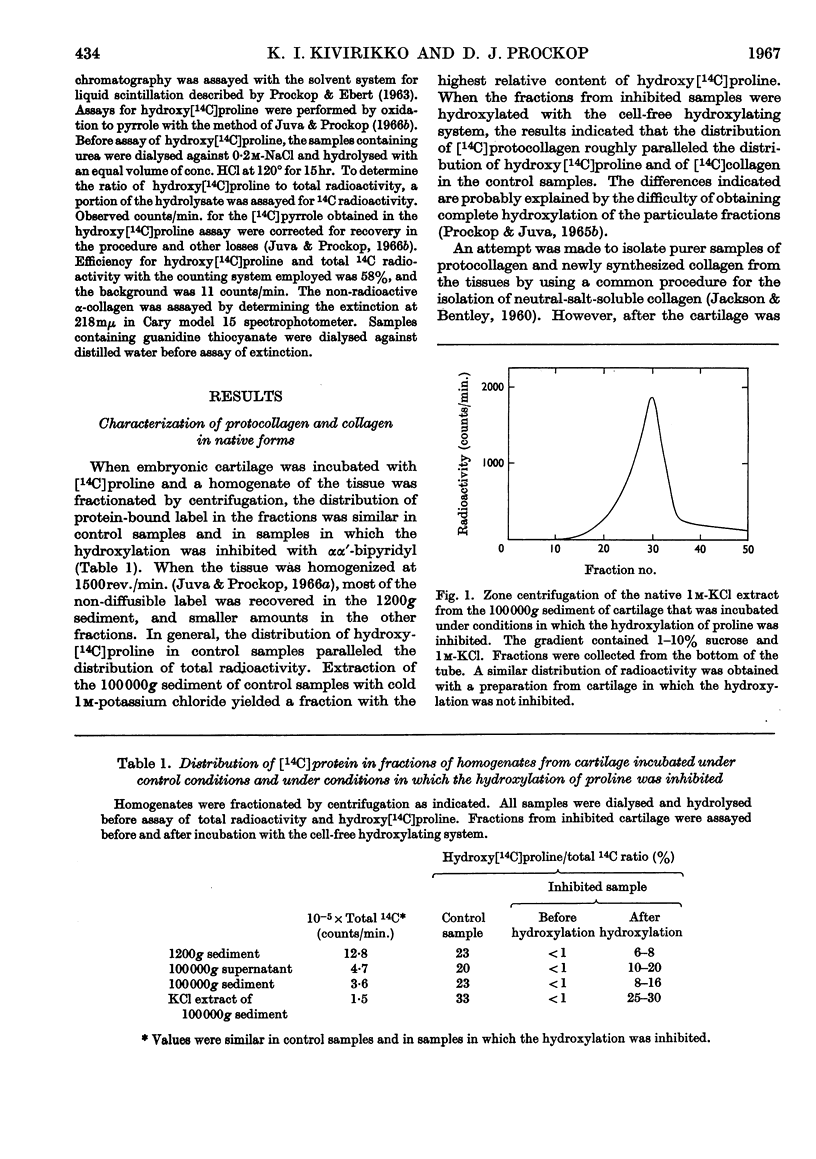
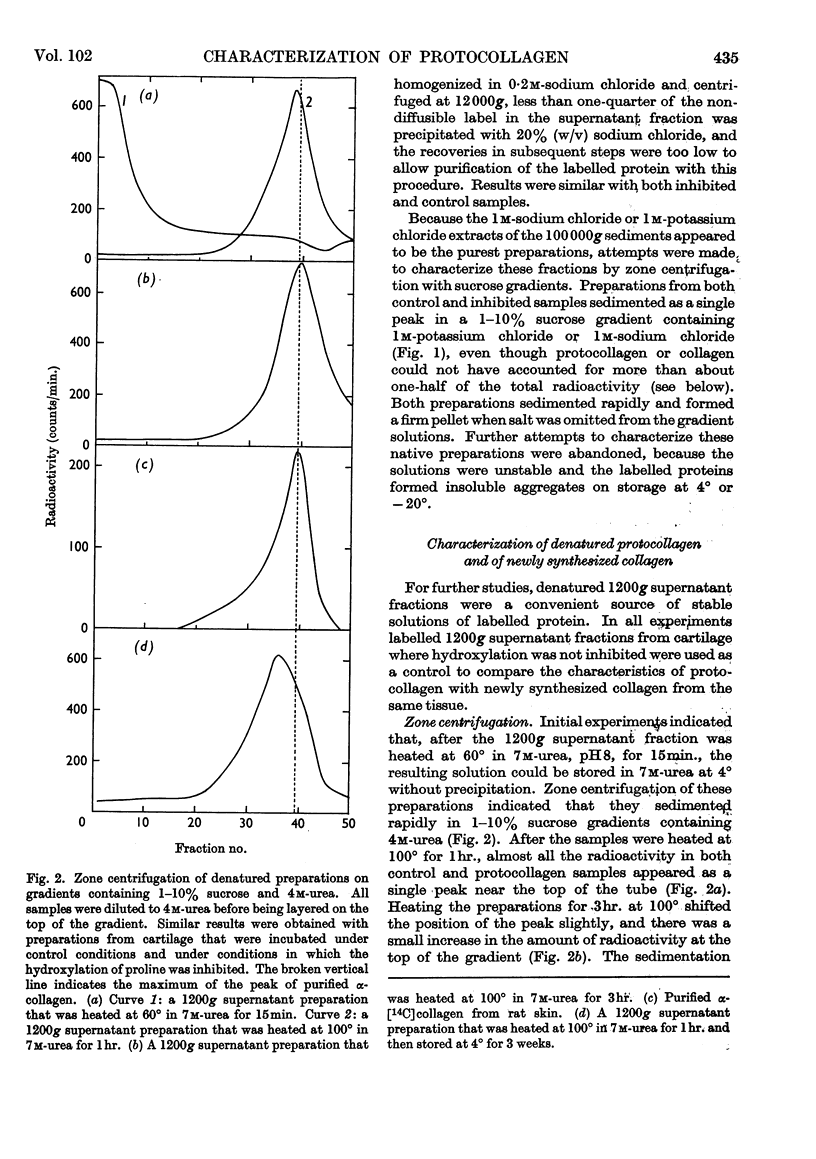
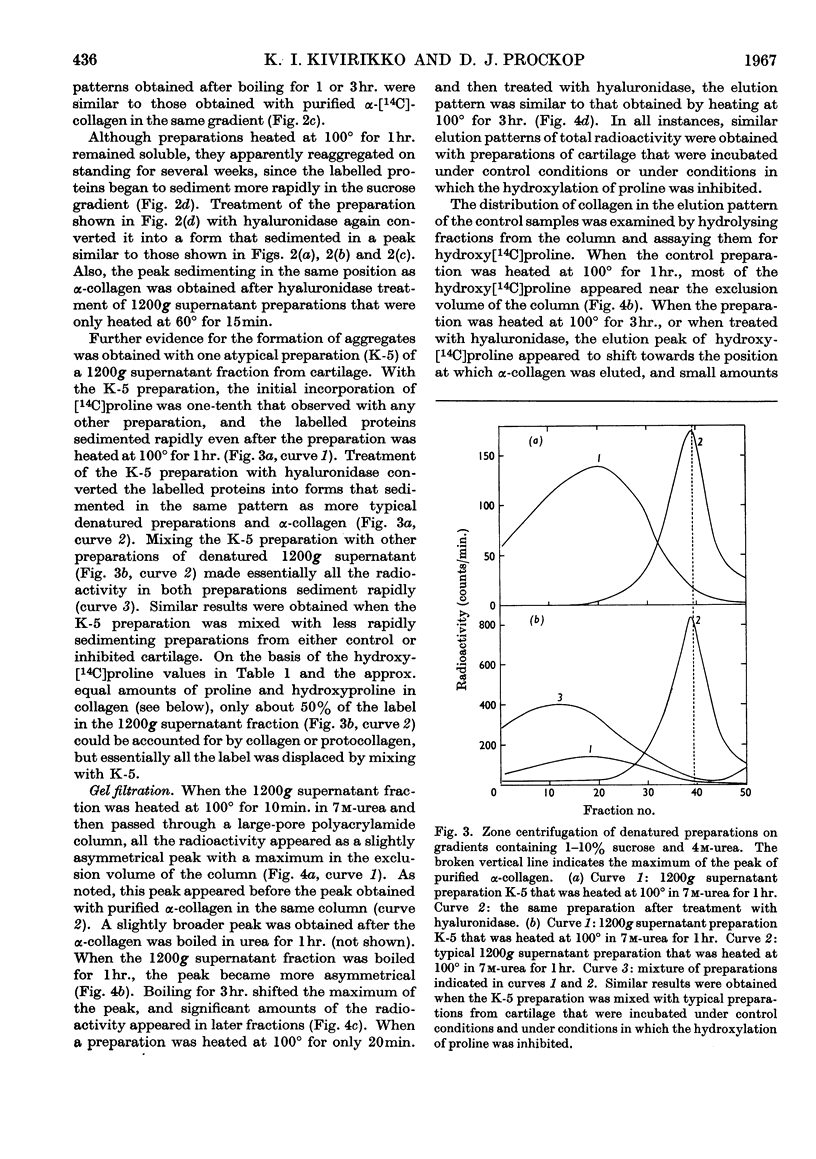
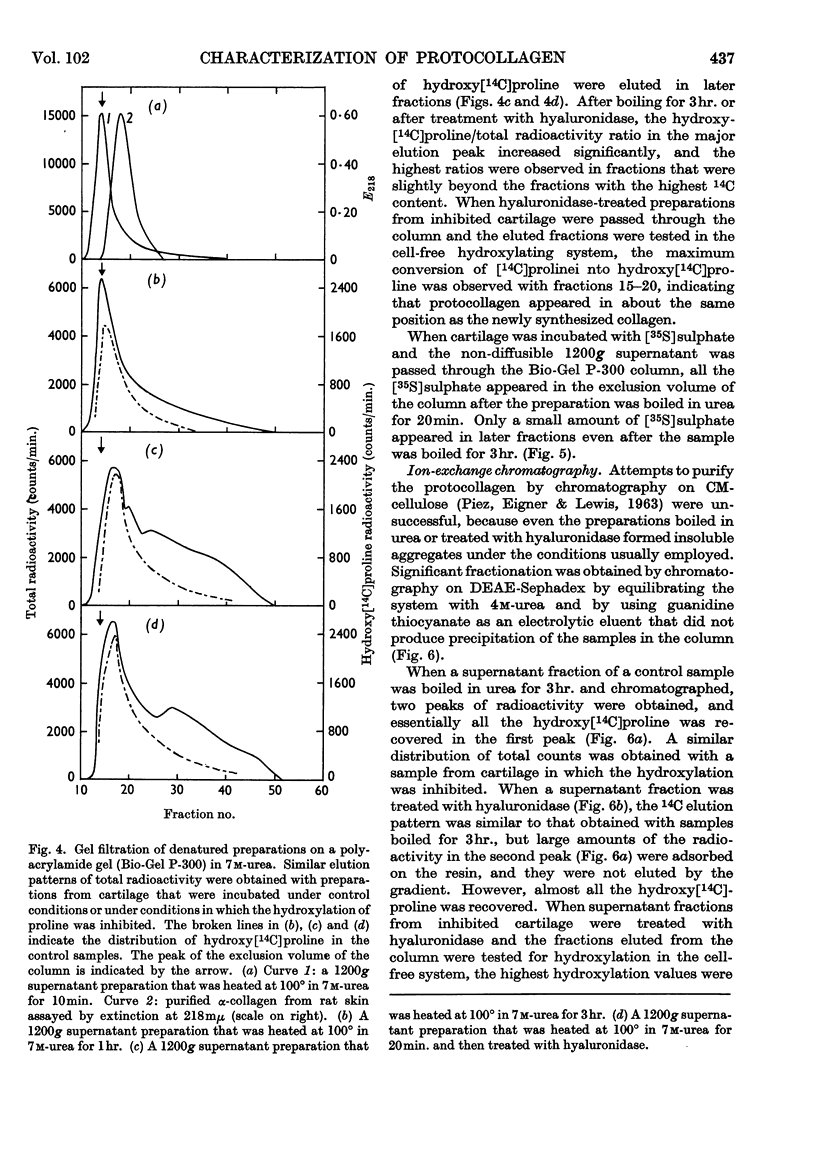
1. Attempts were made to isolate and characterize the protocollagen that accumulates in connective tissue when the hydroxylation of proline and lysine is inhibited. The term protocollagen has been used to describe the proline-rich and lysine-rich polypeptide or polypeptides that serve as substrates for the formation of hydroxyproline and hydroxylysine during the synthesis of collagen. 2. Both protocollagen and newly synthesized collagen from embryonic cartilage were isolated as complex aggregates, which contained sulphated mucopolysaccharides and other proteins or polypeptides from the same tissue. The complexes containing protocollagen were similar to those containing newly synthesized collagen when examined with several different techniques. 3. After the complexes were denatured and disaggregated, zone centrifugation and gel filtration indicated that the denatured protocollagen was similar to the denatured newly synthesized collagen obtained from cartilage in which the hydroxylation was not inhibited, and it was also similar to purified α-collagen. The results suggest that, when the hydroxylation is inhibited, most of the protocollagen polypeptides that accumulate are as large as complete α-chains of collagen. 4. Significant purification of the protocollagen polypeptides was obtained with a new technique for DEAE-Sephadex chromatography in which urea was used to prevent aggregation of the samples and the column was eluted with guanidine thiocyanate. 5. Protocollagen polypeptides were completely hydrolysed to diffusible peptides by a specific collagenase. 6. It is not entirely clear whether the hydroxylation normally begins while relatively short protocollagen molecules are still attached to polysomes, or whether protocollagen molecules of the size of α-collagen are synthesized even when the hydroxylation is not inhibited. 7. Results obtained with puromycin suggest that some hydroxylation occurs with smaller polypeptides, but polypeptide chains approaching the size of α-collagen are required to obtain complete hydroxylation of the appropriate amino acid residues of protocollagen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujimoto D., Tamiya N. Incorporation of O from air into hydroxyproline by chick embryo. Biochem J. 1962 Aug;84(2):333–335. doi: 10.1042/bj0840333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEB A. A., PETERKOFSKY B., UDENFRIEND S. USE OF COLLAGENASE TO IDENTIFY ENZYMATICALLY FORMED COLLAGEN AND A HYDROXYPROLINE-DEFICIENT INTERMEDIATE. J Biol Chem. 1965 Jul;240:3099–3103. [PubMed] [Google Scholar]
- HARRINGTON W. F., VON HIPPEL P. H. The structure of collagen and gelatin. Adv Protein Chem. 1961;16:1–138. doi: 10.1016/s0065-3233(08)60028-5. [DOI] [PubMed] [Google Scholar]
- HURYCH J., SHVAPIL M. INFLUENCE OF CHELATING AGENTS ON THE BIOSYNTHESIS OF COLLAGEN. Biochim Biophys Acta. 1965 Feb 15;97:361–363. doi: 10.1016/0304-4165(65)90108-x. [DOI] [PubMed] [Google Scholar]
- JACKSON D. S., BENTLEY J. P. On the significance of the extractable collagens. J Biophys Biochem Cytol. 1960 Feb;7:37–42. doi: 10.1083/jcb.7.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON D. S., WATKINS D., WINKLER A. FORMATION OF S-RNA HYDROXYPROLINE IN CHICK-EMBRYO AND WOUND GRANULATION TISSUE. Biochim Biophys Acta. 1964 May 18;87:152–153. doi: 10.1016/0926-6550(64)90055-6. [DOI] [PubMed] [Google Scholar]
- JUVA K., PROCKOP D. J. PUROMYCIN INHIBITION OF COLLAGEN SYNTHESIS AS EVIDENCE FOR A RIBOSOMAT OR POST-RIBOSOMAL SITE FOR THE HYDROXYLATION OF PROLINE. Biochim Biophys Acta. 1964 Sep 11;91:174–176. doi: 10.1016/0926-6550(64)90186-0. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. An effect of puromycin on the synthesis of collagen by embryonic cartilage in vitro. J Biol Chem. 1966 Oct 10;241(19):4419–4425. [PubMed] [Google Scholar]
- Juva K., Prockop D. J., Cooper G. W., Lash J. W. Hydroxylation of proline and the intracellular accumulation of a polypeptide precursor of collagen. Science. 1966 Apr 1;152(3718):92–94. doi: 10.1126/science.152.3718.92. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- LEVENE C. I., GROSS J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J Exp Med. 1959 Nov 1;110:771–790. doi: 10.1084/jem.110.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUKENS L. N. EVIDENCE FOR THE NATURE OF THE PRECURSOR THAT IS HYDROXYLATED DURING THE BIOSYNTHESIS OF COLLAGEN HYDROXYPROLINE. J Biol Chem. 1965 Apr;240:1661–1669. [PubMed] [Google Scholar]
- MANDL I., KELLER S., MANAHAN J. MULTIPLICITY OF CLOSTRIDIUM HISTOLYTICUM COLLAGENASES. Biochemistry. 1964 Nov;3:1737–1741. doi: 10.1021/bi00899a026. [DOI] [PubMed] [Google Scholar]
- Manning J. M., Meister A. Conversion of proline to collagen hydroxyproline. Biochemistry. 1966 Apr;5(4):1154–1165. doi: 10.1021/bi00868a007. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochem J. 1965 Sep;96(3):710–716. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H. Chemistry and metabolism of connective tissue glycosaminoglycans (mucopolysaccharides). Int Rev Connect Tissue Res. 1964;2:101–154. doi: 10.1016/b978-1-4831-6751-0.50009-4. [DOI] [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. CONVERSION OF PROLINE TO COLLAGEN HYDROXYPROLINE IN A CELL-FREE SYSTEM FROM CHICK EMBRYO. J Biol Chem. 1963 Dec;238:3966–3977. [PubMed] [Google Scholar]
- PROCKOP D. J., EBERT P. S. A SIMPLE METHOD FOR DIFFERENTIAL ASSAY OF TRITIUM AND CARBON-14 IN WATER-SOLUBLE BIOLOGICAL MATERIALS. Anal Biochem. 1963 Sep;6:263–271. doi: 10.1016/0003-2697(63)90134-9. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., JUVA K. SYNTHESIS OF HYDROXYPROLINE IN VITRO BY THE HYDROXYLATION OF PROLINE IN A PRECURSOR OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:661–668. doi: 10.1073/pnas.53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D., KAPLAN A., UDENFRIEND S. Oxygen-18 studies on the conversion of proline to collagen hydroxyproline. Arch Biochem Biophys. 1963 Jun;101:499–503. doi: 10.1016/0003-9861(63)90509-5. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Weinstein E., Mulveny T. Hydroxylation of lysine in a polypeptide precursor of collagen. Biochem Biophys Res Commun. 1966 Jan 4;22(1):124–128. doi: 10.1016/0006-291x(66)90613-9. [DOI] [PubMed] [Google Scholar]
- SINEX F. M., VAN SLYKE D. D., CHRISTMAN D. R. The source and state of the hydroxylysine of collagen. II. Failure of free hydroxylysine to serve as a source of the hydroxylysine or lysine of collagen. J Biol Chem. 1959 Apr;234(4):918–921. [PubMed] [Google Scholar]
- STETTEN M. R. Some aspects of the metabolism of hydroxyproline, studied with the aid of isotopic nitrogen. J Biol Chem. 1949 Nov;181(1):31–37. [PubMed] [Google Scholar]
- URIVETZKY M., FREI J. M., MEILMAN E. CELL-FREE COLLAGEN BIOSYNTHESIS AND THE HYDROXYLATION OF SRNA-PROLINE. Arch Biochem Biophys. 1965 Mar;109:480–489. doi: 10.1016/0003-9861(65)90392-9. [DOI] [PubMed] [Google Scholar]


