Abstract
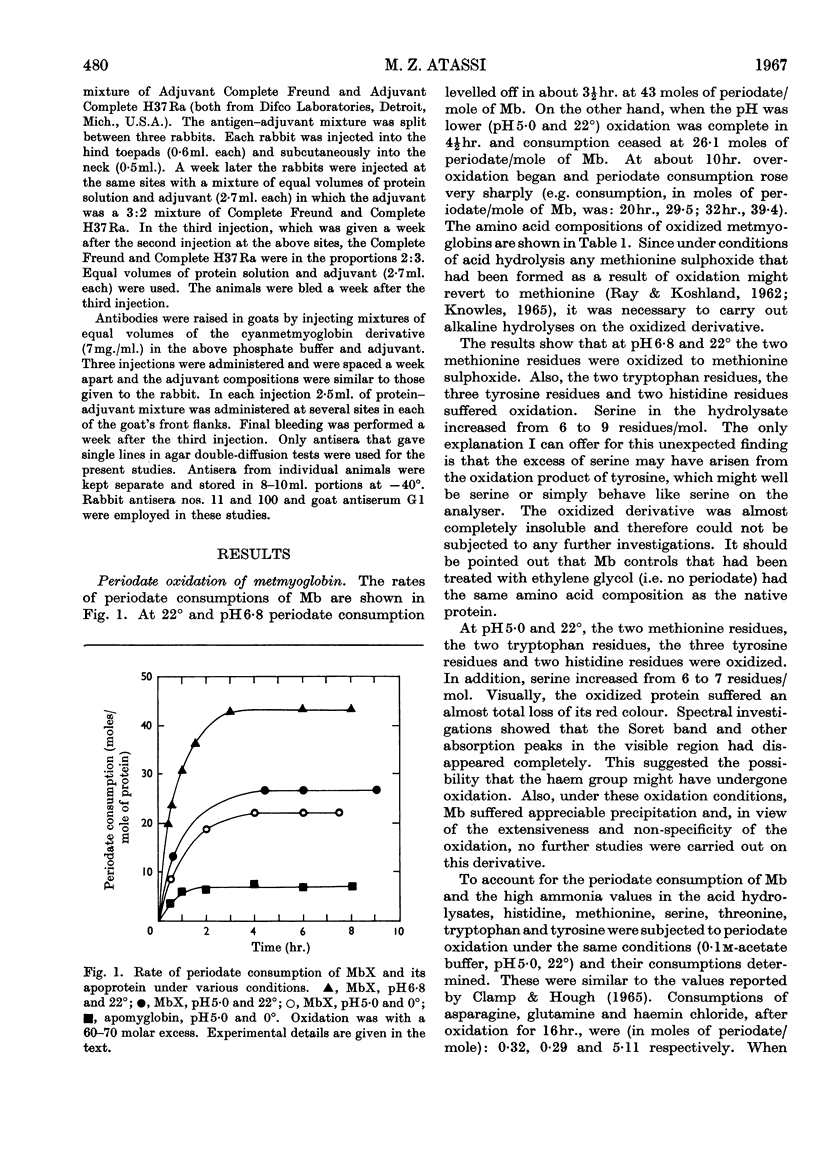
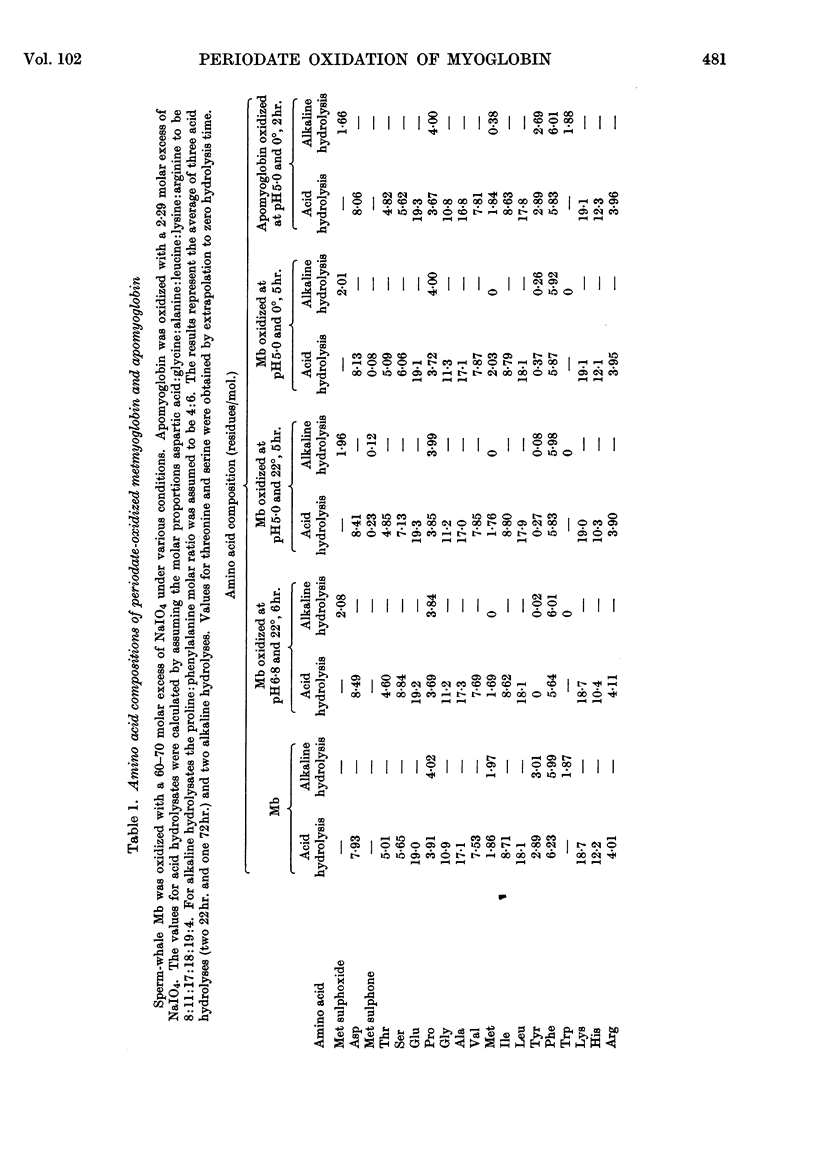
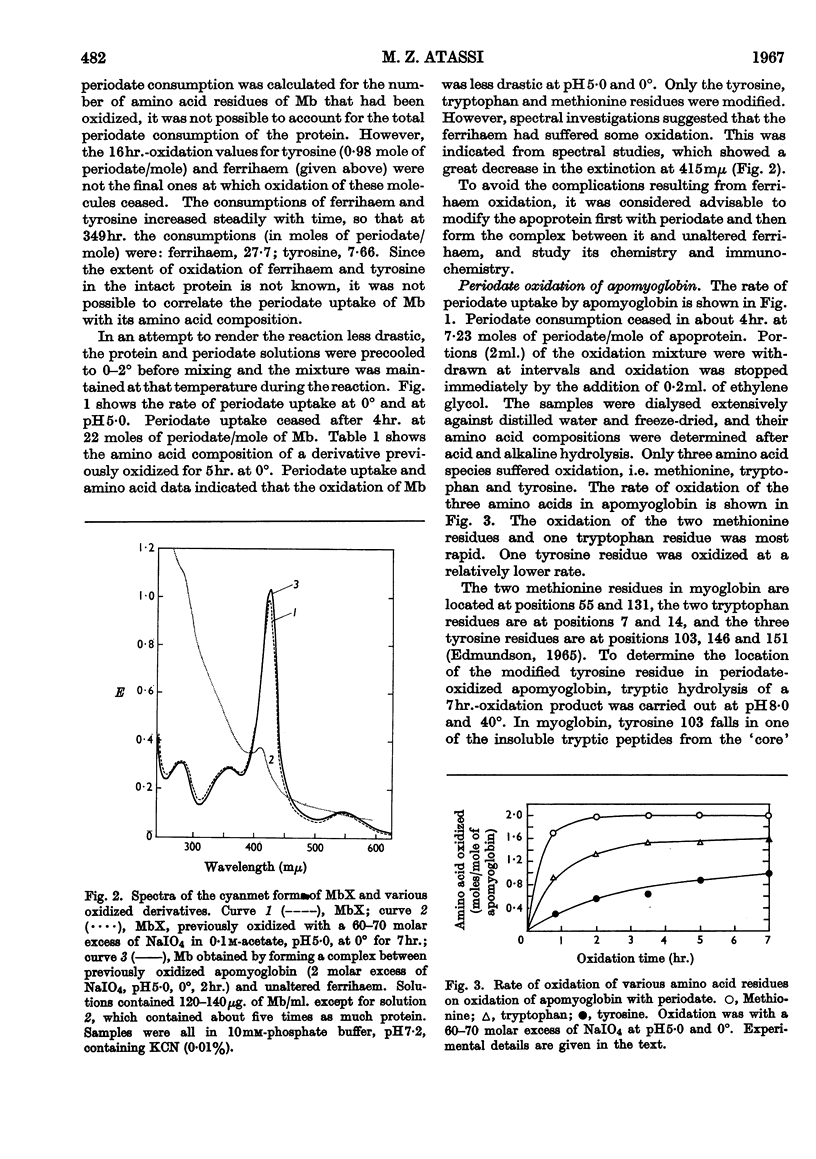
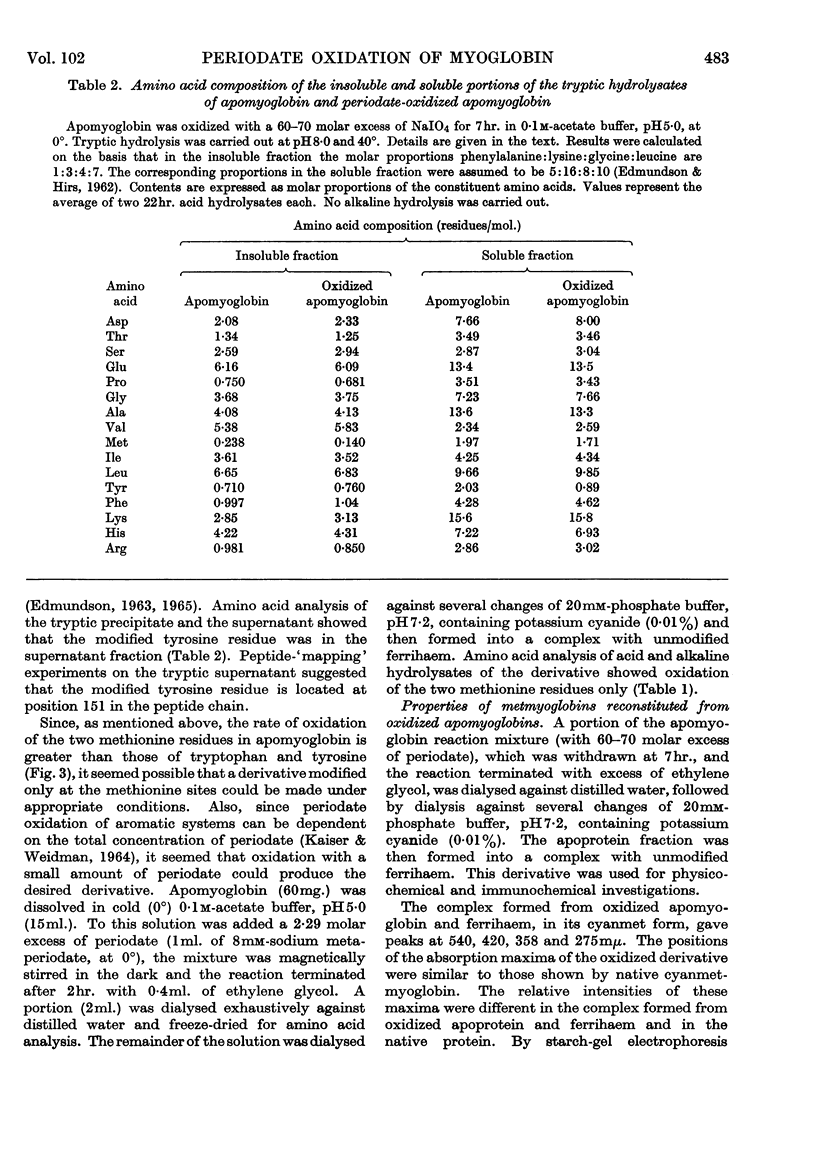
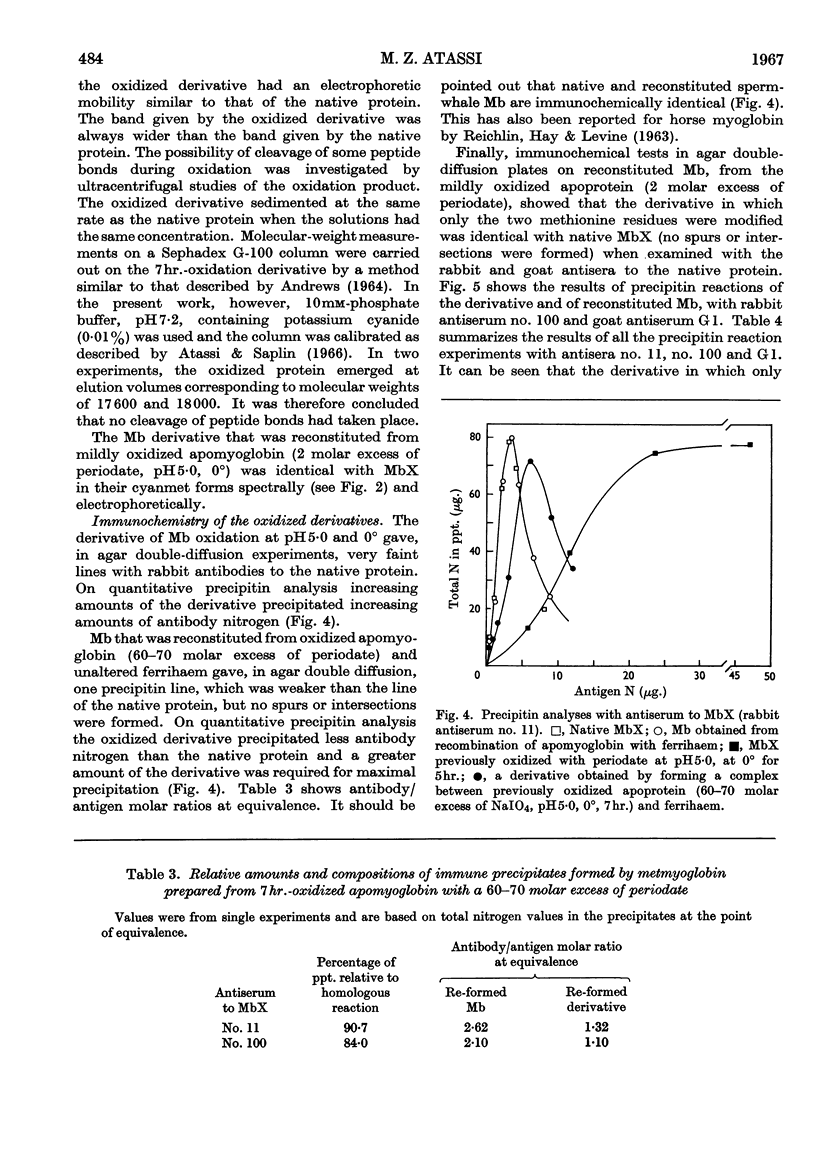
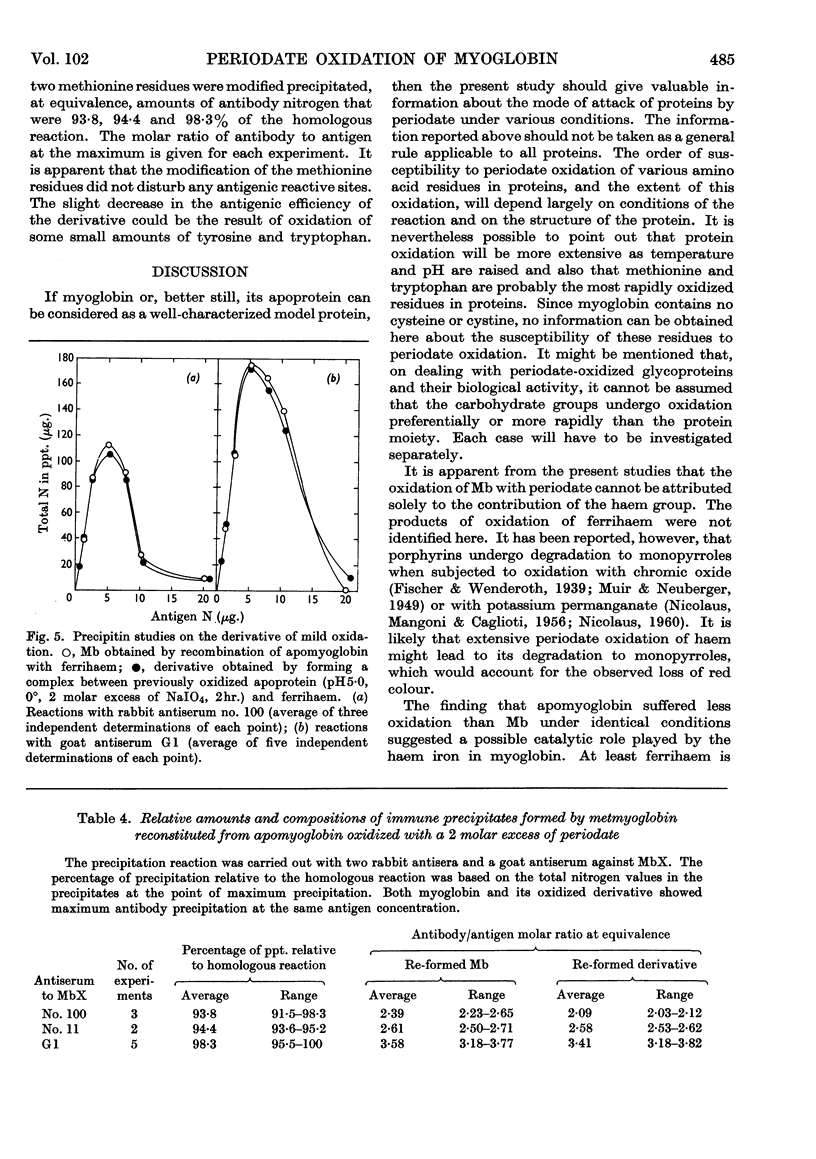
1. Oxidation of sperm-whale metmyoglobin and its apoprotein with periodate has been investigated under various conditions of pH and temperature to find those under which the reagent acted with specificity. 2. At pH6·8 and 22° consumption of periodate ceased in 3½hr. at 43 moles of periodate/mole of myoglobin. The two methionine residues, the two tryptophan residues, the three tyrosine residues and two histidine residues were oxidized; serine increased in the hydrolysates from 6 to 9 residues/mol. 3. At pH5·0 and 22°, consumption levelled off in 4½hr. at 26 moles of periodate/mole of myoglobin and resulted in the modification of the two methionine residues, the two tryptophan residues, the three tyrosine residues and two histidine residues; serine increased from 6 to 7 residues/mol. and, also, ferrihaem suffered considerable oxidation. 4. Oxidation at pH5·0 and 0° resulted at completion (4hr.) in the consumption of 22 moles of periodate/mole of myoglobin and in the modification of the methionine, tyrosine and tryptophan residues. Spectral studies indicated oxidation of the haem group. This derivative reacted very poorly with rabbit antisera to MbX (the major component no. 10 obtained by CM-cellulose chromatography; Atassi, 1964). 5. Oxidation of apomyoglobin at pH5·0 and 0° was complete in 4hr. with the consumption of 7·23 moles of periodate/mole of apoprotein. The rate of oxidation in decreasing order was: methionine; tryptophan; tyrosine; and after 7hr. of reaction the following residues/mol. were oxidized: methionine, 2·0; tryptophan, 1·6; tyrosine, 0·99. No peptide bonds were cleaved. Metmyoglobin prepared from the 7hr.-oxidized apoprotein showed that the reactivity with antisera to MbX had diminished considerably. 6. Milder oxidation of apoprotein (2 molar excess of periodate, pH5·0, 0°, 2hr.) resulted in the modification of 1·66 residues of methionine/mol. Metmyoglobin prepared from this apoprotein was identical with native MbX spectrally, electrophoretically and immunochemically. It was concluded that the methionine residues at positions 55 and 131 were not essential parts of the antigenic sites of metmyoglobin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z. PROPERTIES OF COMPONENTS OF MYOGLOBIN OF THE SPERM WHALE. Nature. 1964 May 2;202:496–498. doi: 10.1038/202496a0. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Brown R. K., McEwan M. Immunochemical studies of hemoglobin and the role of the sulfhydryl groups. Immunochemistry. 1965 Dec;2(4):379–389. doi: 10.1016/0019-2791(65)90037-6. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Role of the amino groups and C-terminal of sperm-whale myoglobin in the antigen-antibody reaction. Nature. 1966 Mar 19;209(5029):1209–1211. doi: 10.1038/2091209a0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Saplin B. J. Studies on myoglobin from the finback whale (Balaenoptera physalus). Preparation, physicochemical and immunochemical characterization, differentiation from sperm-whale myoglobin, amino acid composition and end-terminal analyses. Biochem J. 1966 Jan;98(1):82–93. doi: 10.1042/bj0980082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANASZAK L. J., ANDREWS P. A., BURGNER J. W., EYLAR E. H., GURD F. R. CARBOXYMETHYLATION OF SPERM WHALE METMYOGLOBIN. J Biol Chem. 1963 Oct;238:3307–3314. [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Wilkinson J. M. The immunological activity of some of the chymotryptic peptides of sperm-whale myoglobin. Biochem J. 1965 Mar;94(3):545–556. doi: 10.1042/bj0940545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON H. B. Treatment of corticotrophin with periodate and borohydride. Biochem J. 1962 Apr;83:91–94. doi: 10.1042/bj0830091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction between metmyoglobin and alkyl hydro-peroxides. Biochem J. 1953 Sep;55(2):230–236. doi: 10.1042/bj0550230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction between metmyoglobin and hydrogen peroxide. Biochem J. 1952 Nov;52(3):511–517. doi: 10.1042/bj0520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction of metmyoglobin with strong oxidizing agents. Biochem J. 1954 Oct;58(2):188–195. doi: 10.1042/bj0580188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- KENDREW J. C., WATSON H. C., STRANDBERG B. E., DICKERSON R. E., PHILLIPS D. C., SHORE V. C. The amino-acid sequence x-ray methods, and its correlation with chemical data. Nature. 1961 May 20;190:666–670. doi: 10.1038/190666a0. [DOI] [PubMed] [Google Scholar]
- KING N. K., LOONEY F. D., WINFIELD M. E. MYOGLOBIN FREE-RADICALS. Biochim Biophys Acta. 1964 Jul 29;88:235–236. doi: 10.1016/0926-6577(64)90179-2. [DOI] [PubMed] [Google Scholar]
- KNOWLES J. R. THE ROLE OF METHIONINE IN ALPHA-CHYMOTRYPSIN-CATALYSED REACTIONS. Biochem J. 1965 Apr;95:180–190. doi: 10.1042/bj0950180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, STRUMEYER D. H., RAY W. J., Jr Amino acids involved in the action of chymotrypsin. Brookhaven Symp Biol. 1962 Dec;15:101–133. [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H. M., Neuberger A. The biogenesis of porphyrins. The distribution of N in the ring system. Biochem J. 1949;45(2):163–170. [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Identification of amino acids involved in phosphoglucomutase action. J Biol Chem. 1962 Aug;237:2493–2505. [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. IMMUNOCHEMICAL STUDIES OF HEMOGLOBIN AND MYOGLOBIN AND THEIR GLOBIN MOIETIES. Biochemistry. 1963 Sep-Oct;2:971–979. doi: 10.1021/bi00905a013. [DOI] [PubMed] [Google Scholar]


