Abstract
Combinations of carbapenems and epigallocatechin gallate (EGCg; a main constituent of tea catechins) showed potent synergy against 24 clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). MICs of imipenem in the presence of EGCg at 3.125, 6.25, 12.5, and 25 μg/ml, were restored to the susceptible breakpoint (≤4 μg/ml) for 8, 38, 46, and 75% of the MRSA isolates, respectively. Similar results were also observed for combinations of panipenem or meropenem and EGCg. Therefore, the combinations may be worthy of further evaluation in vivo against MRSA infection.
Methicillin-resistant Staphylococcus aureus (MRSA) has become a major nosocomial pathogen in the past 2 decades. Therapeutic options for MRSA infection are very limited because most MRSA strains are resistant not only to β-lactams but also to multiple antimicrobial agents, such as macrolides, aminoglycosides, and fluoroquinolones (8). The emergence of MRSA strains with reduced susceptibility to vancomycin suggests that MRSA may eventually become fully resistant to vancomycin (1, 2). Therefore, new chemotherapeutic agents and new approaches are urgently needed to combat such multiple-antibiotic-resistant bacteria.
Carbapenems are relatively new β-lactams that have a broad spectrum and strong activity against many pathogens. However, the carbapenems (imipenem [IPM], panipenem [PAPM], and meropenem [MEM]) do not show high levels of activity against MRSA.
It has been previously demonstrated that epigallocatechin gallate (EGCg; a main constituent of tea catechins) is able to act synergistically with β-lactams against MRSA (5, 12). Two groups using an aqueous extract of tea further confirmed the synergy between tea and β-lactams (14, 15). It was recently demonstrated that the EGCg-induced damage of the bacterial cell wall and interference with its integrity through direct binding with peptidoglycan are responsible for the synergism between EGCg and β-lactams against MRSA (16).
In determining the possible clinical use of EGCg against MRSA infections, the bioavailable concentration of EGCg in vivo is the most crucial factor. In this study, therefore, we examined carbapenems in combination with the lowest possible concentrations of EGCg in vitro.
EGCg was extracted from green tea, and the purity of the EGCg was confirmed to be 98% by high-performance liquid chromatography analysis. The following antibiotics were used: IPM (Banyu Pharmaceutical Co., Tokyo, Japan), PAPM (Sankyo Organic Chemicals Co., Kawasaki, Japan), and MEM (Sumitomo Pharmaceuticals Co., Osaka, Japan).
The twenty-four clinical isolates of MRSA used were from specimens submitted for routine culture at the clinical microbiology laboratories of Showa University Hospital. All 24 isolates carried the mecA gene, which was confirmed by PCR analysis as described previously (6, 16). Mueller-Hinton broth supplemented with 25 mg of Ca2+/liter, 12.5 mg of Mg2+/liter, and 2% NaCl was used for susceptibility tests and time-kill assays. MICs were determined by the broth microdilution and checkerboard methods (11) with a final inoculum concentration of >5 × 105 CFU/ml. After stationary incubation of the isolates at 35°C for 24 h, the lowest concentration of the twofold-serial-diluted antibiotic(s) and/or EGCg at which no visible growth occurred was defined as the antibiotic's or EGCg’s MIC. The bacteria were also cultured in 3 ml of Mueller-Hinton broth, and their growth was detected with a spectrophotometer (optical density at 600 nm) at 24 h. Synergy was measured by a fractional inhibitory concentration (FIC) index. The FIC of the combination was calculated by dividing the MIC of the antibiotic-EGCg combination by the MIC of the antibiotic or of EGCg alone, and the FIC index was obtained by adding the FIC of the antibiotic and that of EGCg. The FIC index results were interpreted as follows: ≤0.5, synergy; >0.5 to 1, additive effect; and >1 to 2, no effect. In the killing curves, synergy was defined as present when the decrease in CFU per milliliter between results of treatment with the combination and those of its most active constituent after 24 h was ≥2-log10 and when the number of surviving organisms after treatment with the combination of β-lactams and EGCg at their sub-MICs was ≥2-log10 CFU/ml below the starting concentration of inoculum. The data from time-kill assays are presented as the means ± standard deviations (see Fig. 1).
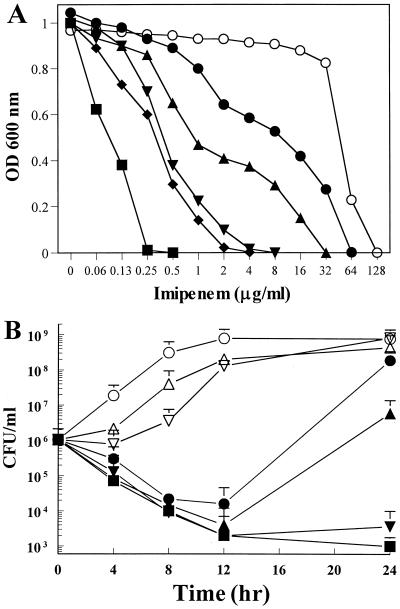
FIG. 1.
Synergistic anti-MRSA effects of combinations of IPM and EGCg. (A) Growth inhibition curves. ○, IPM alone; •, IPM plus EGCg at 1.56 μg/ml; ▴, IPM plus EGCg at 3.12 μg/ml; ▾, IPM plus EGCg at 6.25 μg/ml; ⧫, IPM plus EGCg at 12.5 μg/ml; ▪, IPM plus EGCg at 25 μg/ml; OD, optical density. (B) Time-kill curves. ○, no addition; ▵, IPM at 32 μg/ml alone; ▿, EGCg at 25 μg/ml alone; •, IPM at 4 μg/ml plus EGCg at 3.125 μg/ml (1/32 of their MICs); ▴, IPM at 8 μg/ml plus EGCg at 6.25 μg/ml (1/16 of their MICs); ▾, IPM at 16 μg/ml plus EGCg at 12.5 μg/ml (1/8 of their MICs); ▪, IPM at 32 μg/ml plus EGCg at 25 μg/ml (1/4 of their MICs).
The MIC of EGCg alone for the MRSA isolates was 100 μg/ml. Synergy was observed when β-lactams were combined with EGCg at a concentration of less than one-fourth the MIC of EGCg alone. Among the tested β-lactams, which included penicillins (benzylpenicillin, ampicillin, oxacillin, and methicillin), cephems (cephalexin, cefmetazole, and cefotaxime), and carbapenems (IPM, PAPM, and MEM), the combinations between carbapenems and EGCg showed the most potent synergy.
The effects of combination of carbapenems and EGCg are summarized in Table 1. The synergistic or additive effects were observed to occur against 100% of the MRSA strains tested in a dose-dependent manner. The MIC at which 50% of the isolates treated with IPM were inhibited decreased from 128 μg/ml to 64, 32, 8, 8, and 0.25 μg/ml when in combination with 1.56, 3.125, 6.25, 12.5, and 25 μg of EGCg/ml, respectively. Furthermore, the MICs of IPM in the presence of EGCg at 3.125, 6.25, 12.5, and 25 μg/ml were restored to the susceptibility breakpoint (≤4 μg/ml) for 8, 38, 46, and 75% of MRSA isolates, respectively.
TABLE 1.
Effects of carbapenems in combination with EGCg against 24 MRSA isolates
| Combination | MIC50 (μg/ml)a of combination | % of MRSA isolates showing indicated effect:
|
|||
|---|---|---|---|---|---|
| Synergistic | Additive | None | MIC of ≤4 μg/mlb | ||
| EGCg 1.56 μg/ml | |||||
| IPM | 64 | 0 | 46 | 54 | 0 |
| PAPM | 32 | 4 | 33 | 63 | 4 |
| MEM | 64 | 0 | 42 | 58 | 0 |
| EGCg 3.125 μg/ml | |||||
| IPM | 32 | 42 | 41 | 17 | 8 |
| PAPM | 16 | 29 | 63 | 8 | 21 |
| MEM | 32 | 38 | 62 | 0 | 4 |
| EGCg 6.25 μg/ml | |||||
| IPM | 8 | 83 | 17 | 0 | 38 |
| PAPM | 8 | 75 | 17 | 8 | 46 |
| MEM | 8 | 83 | 17 | 0 | 25 |
| EGCg 12.5 μg/ml | |||||
| IPM | 8 | 88 | 12 | 0 | 46 |
| PAPM | 2 | 83 | 9 | 8 | 67 |
| MEM | 8 | 88 | 12 | 0 | 42 |
| EGCg 25 μg/ml | |||||
| IPM | 0.25 | 92 | 8 | 0 | 75 |
| PAPM | 0.5 | 88 | 12 | 0 | 75 |
| MEM | 2 | 83 | 17 | 0 | 71 |
MICs at which 50% of the isolates treated with carbapenem alone are inhibited: IPM, 128 μg/ml; PAPM 32 μg/ml; MEM, 64 μg/ml.
MIC susceptibility breakpoint is ≤4 μg/ml for IPM and MEM, as determined by NCCLS, but is not available for PAPM at present.
Figure 1Ashows the results for the IPM-EGCg combination against strain F-74, which has a high level of resistance to carbapenems (MIC, 128 μg/ml). The growth of F-74 was inhibited by EGCg even at the low concentration of 1.56 μg/ml. Figure 1B, shows the results of time-kill assays of strain F-74. The combination of 32 μg of IPM/ml and 25 μg of EGCg/ml (concentrations that are one-fourth their MICs) showed synergistic bactericidal activity, resulting after 24 h in a >2-log10 decrease in CFU per milliliter from that resulting from treatment with IPM or EGCg alone and from the starting inoculum. PAPM-EGCg and MEM-EGCg combinations also showed similar results (data not shown).
Tea, one of the most popular beverages in the world, is consumed every day by billions of people. Capsules of tea catechins and EGCg are becoming available for research and preclinical trials. The safe consumption of tea for thousands of years indicates the low toxicity of tea and EGCg. However, it is hard to predict synergistic effects in vivo on the basis of the presented in vitro evidence alone because it is difficult to estimate the in vivo concentration of EGCg, especially the bioavailable concentration of free (active) EGCg, after tea has been drunk or EGCg capsules have been ingested.
Recently, researchers began to pay attention to the effects of the pharmacokinetics of EGCg and tea catechins on their absorption, distribution, and elimination in animals and humans (3, 4, 7, 9, 10, 13, 17). Usually the EGCg concentration in tea is 2 to 3 mg/ml. EGCg is absorbed through the digestive tract and distributed to many organs in both animals and humans. EGCg at 5.6 μg/ml in rat blood plasma was detected after the administration of EGCg at 500 mg/kg of body weight (9). Total catechins, including epicatechin, epicatechin gallate, and EGCg, were detected in rat blood plasma at 15 to 112 μg/ml at 2 h after oral administration of catechins at 5,000 mg/kg (17). EGCg was detected at 2 μg/ml in human blood plasma 90 min after 525 mg of EGCg in capsule form had been ingested (10). Therefore, the combination of carbapenems and EGCg against MRSA may be worthy of further evaluation in vivo.
REFERENCES
- 1.Centers for Disease Control and Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:813-815. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—Japan, 1996. Morb. Mortal. Wkly. Rep. 46:624-635. [PubMed] [Google Scholar]
- 3.Chen, L., M. J. Lee, H. Li, and C. S. Yang. 1997. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 25:1045-1050. [PubMed] [Google Scholar]
- 4.Hamilton-Miller, J. M. T. 1995. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 39:2375-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, Z.-Q., W.-H. Zhao, Y. Hara, and T. Shimamura. 2001. Epigallocatechin gallate synergy with ampicillin-sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 48:361-364. [DOI] [PubMed] [Google Scholar]
- 6.Kohner, P., J. Uhl, C. Kolbert, D. Persing, and F. Cockerill III. 1999. Comparison of susceptibility testing methods with mecA gene analysis for determining oxacillin (methicillin) resistance in clinical isolates of Staphylococcus aureus and coagulase-negative Staphylococcus spp. J. Clin. Microbiol. 37:2952-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, M. J., Z. Y. Wang, H. Li, L. Chen, Y. Sun, S. Gobbo, D. A. Balentine, and C. S. Yang. 1995. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomark. Prev. 4:393-399. [PubMed] [Google Scholar]
- 8.Maple, P. A. C., J. M. T. Hamilton-Miller, and W. Brumfitt. 1989. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet i:537-540. [DOI] [PubMed]
- 9.Nakagawa, K., and T. Miyazawa. 1997. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 43:679-684. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa, K., S. Okuda, and T. Miyazawa. 1997. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 61:1981-1985. [DOI] [PubMed] [Google Scholar]
- 11.Norden, C. W., H. Wentzel, and E. Keleti. 1979. Comparison of techniques for measurement of in vitro antibiotic synergism. J. Infect. Dis. 140:629-633. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi, A., Z. Cai, M. Toda, Y. Hara, and T. Shimamura. 1995. Appearance of antibacterial activity of oxacillin against methicillin resistant Staphylococcus aureus (MRSA) in the presence of catechin. J. Jpn. Assoc. Infect. Dis. 69:1126-1134. (In Japanese.) [DOI] [PubMed]
- 13.Unno, T., K. Kondo, H. Itakura, and T. Takeo. 1996. Analysis of (−)-epigallocatechin gallate in human serum obtained after ingesting green tea. Biosci. Biotechnol. Biochem. 60:2066-2068. [DOI] [PubMed] [Google Scholar]
- 14.Yam, T. S., J. M. T. Hamilton-Miller, and S. Shah. 1998. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2" synthesis, and β-lactamase production in Staphylococcus aureus. J. Antimicrob. Chemother. 42:211-216. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki, K. 1996. Enhancing effect of Japanese green tea extract on the growth-inhibitory activity of antibiotics against clinically isolated MRSA strains. Jpn. J. Chemother. 44:477-482. (In Japanese.)
- 16.Zhao, W.-H., Z.-Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu, M., Y. Chen, and R. C. Li. 2000. Oral absorption and bioavailability of tea catechins. Planta Med. 66:444-447. [DOI] [PubMed] [Google Scholar]