Abstract
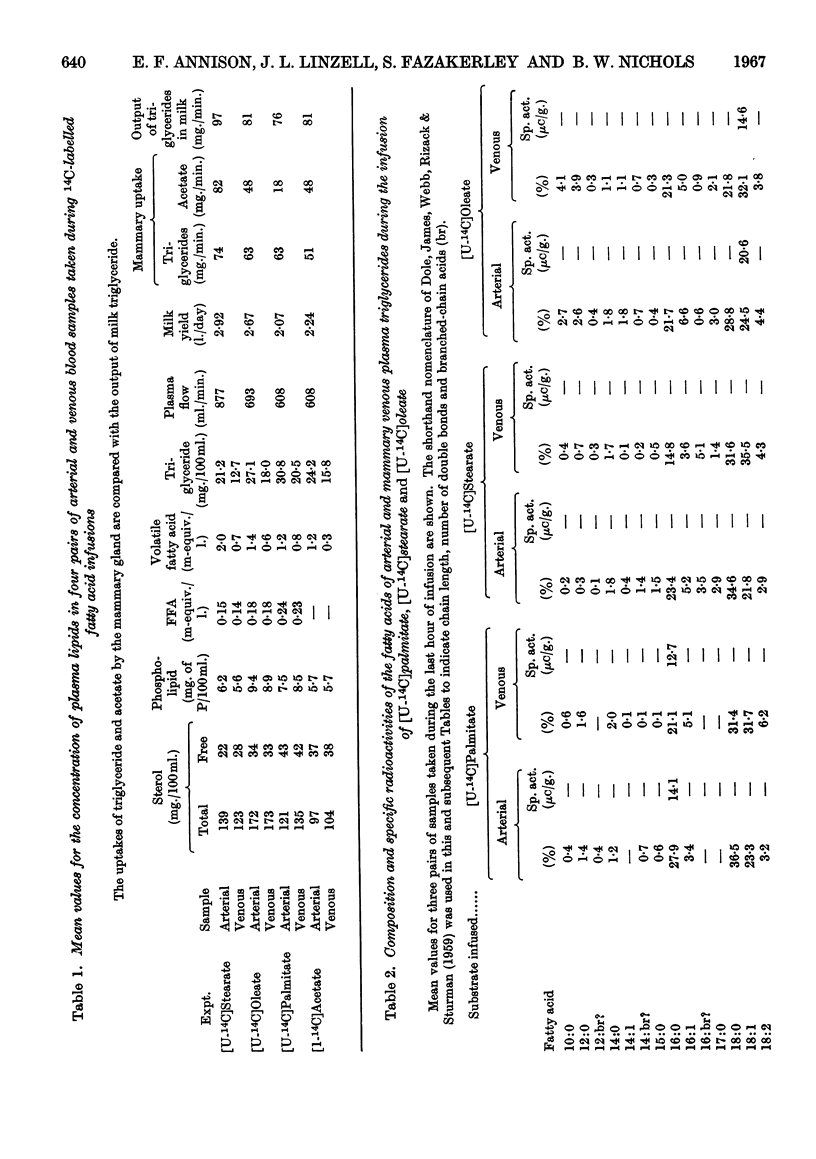
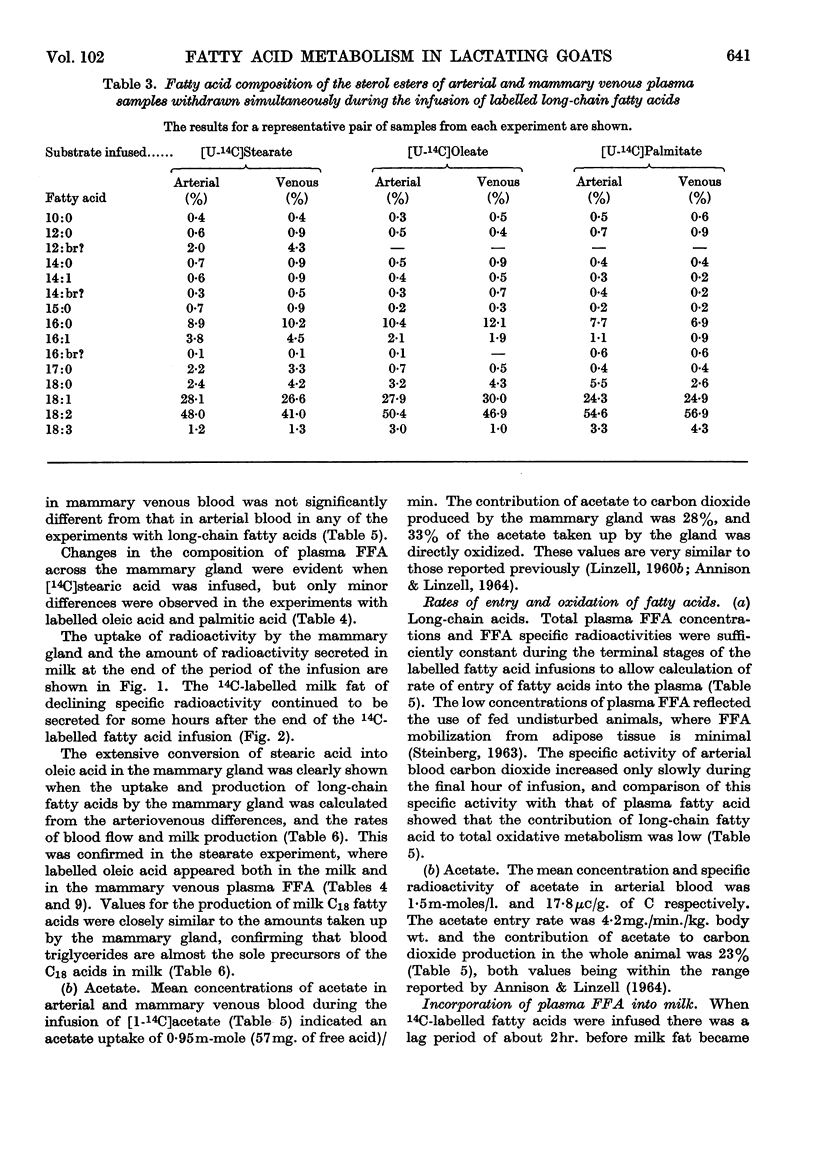
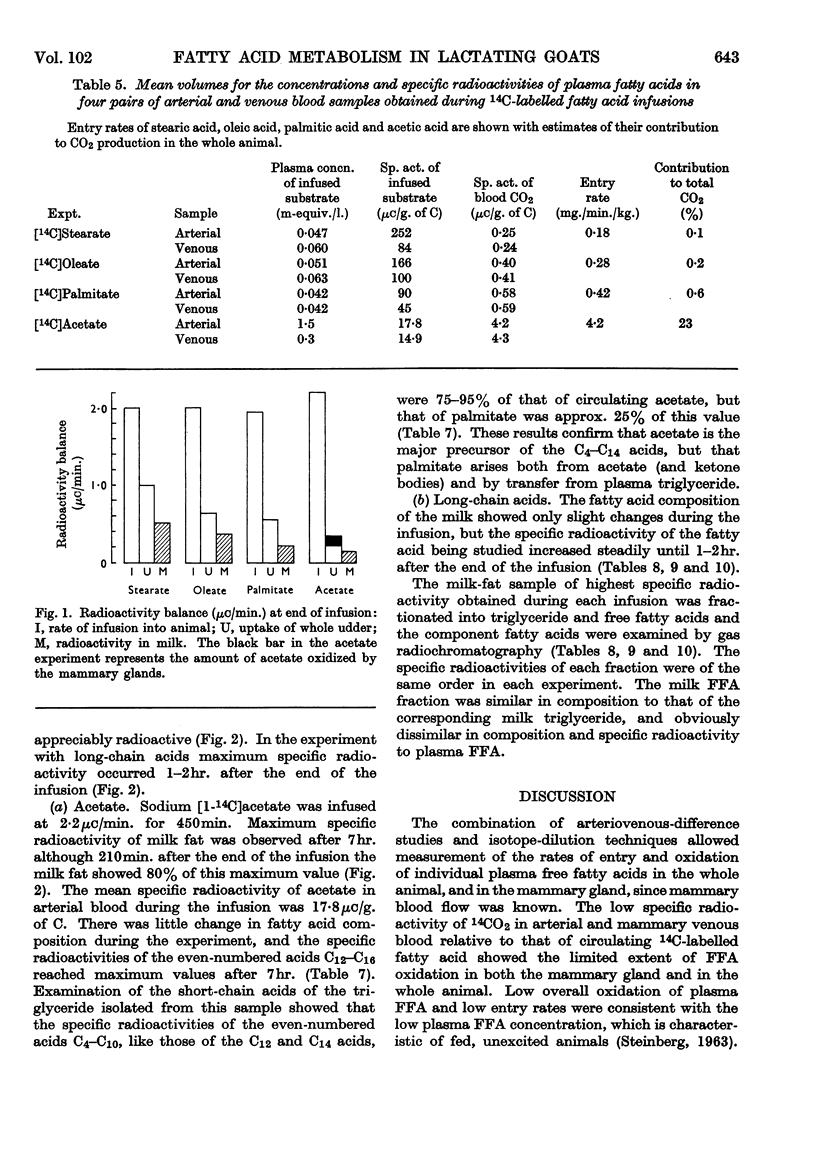
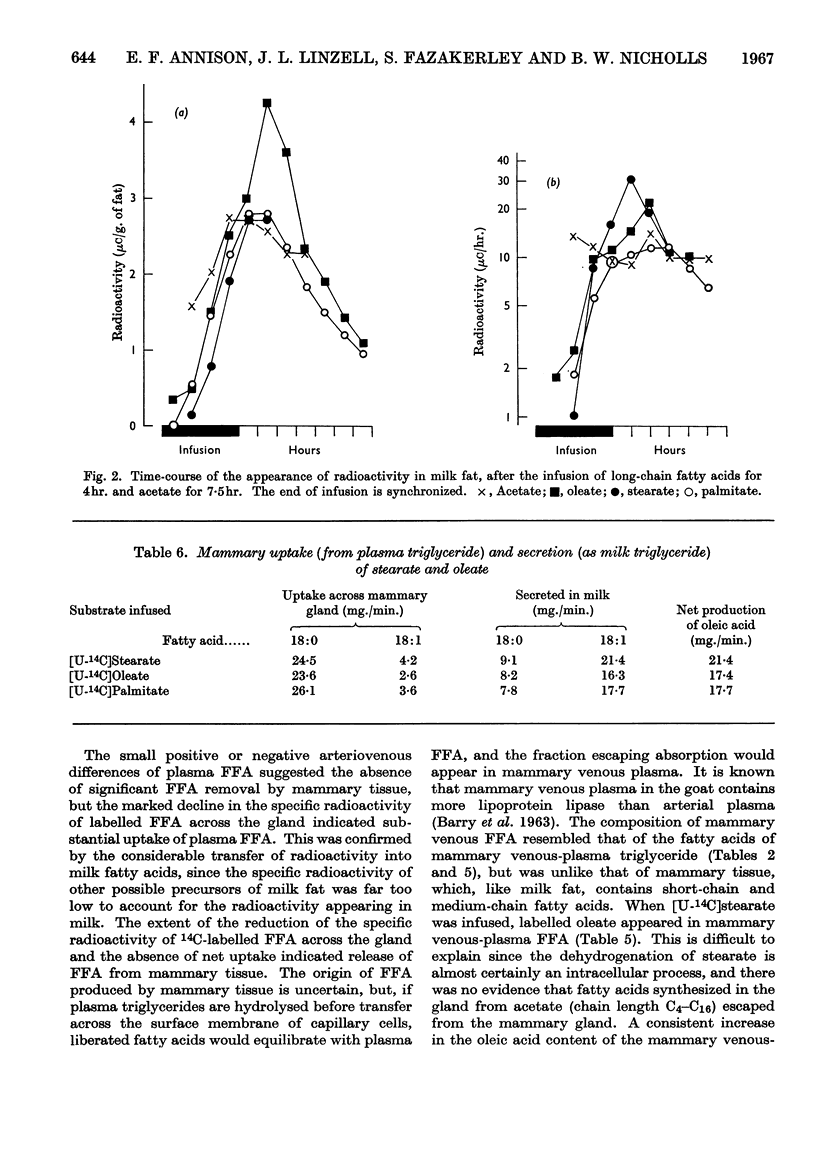
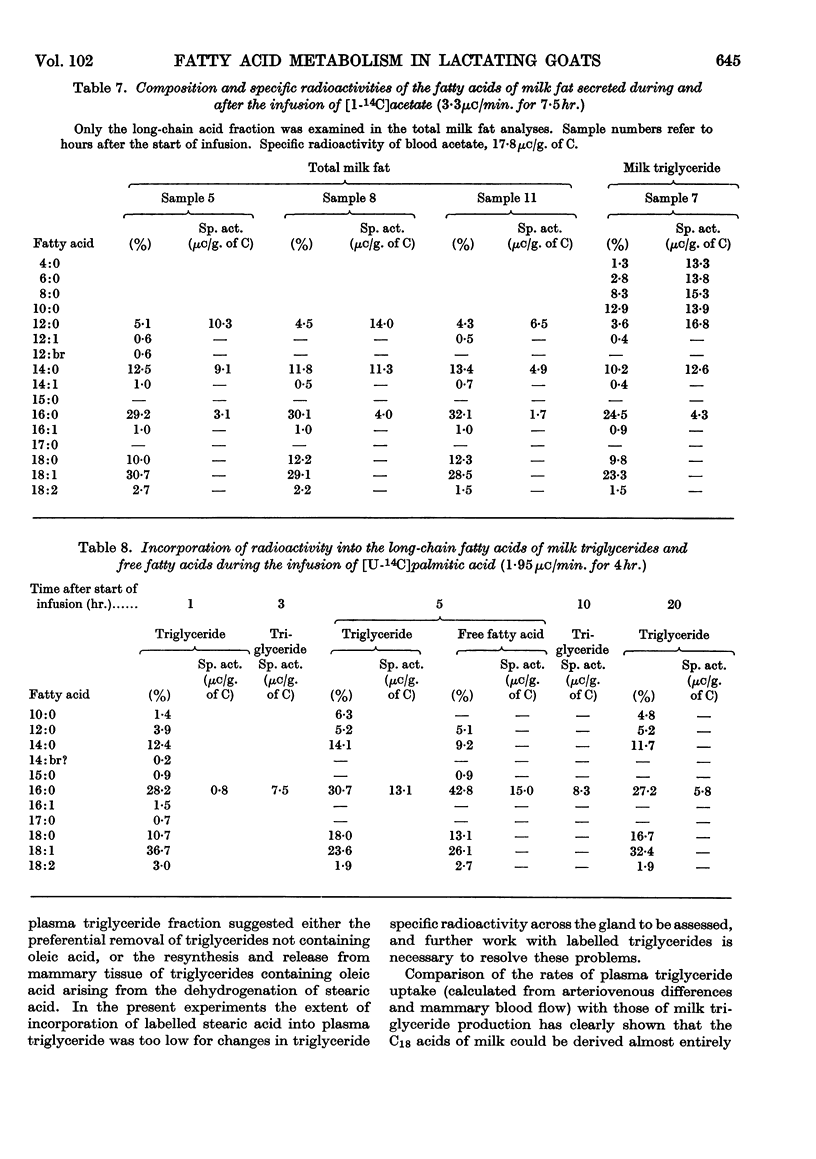
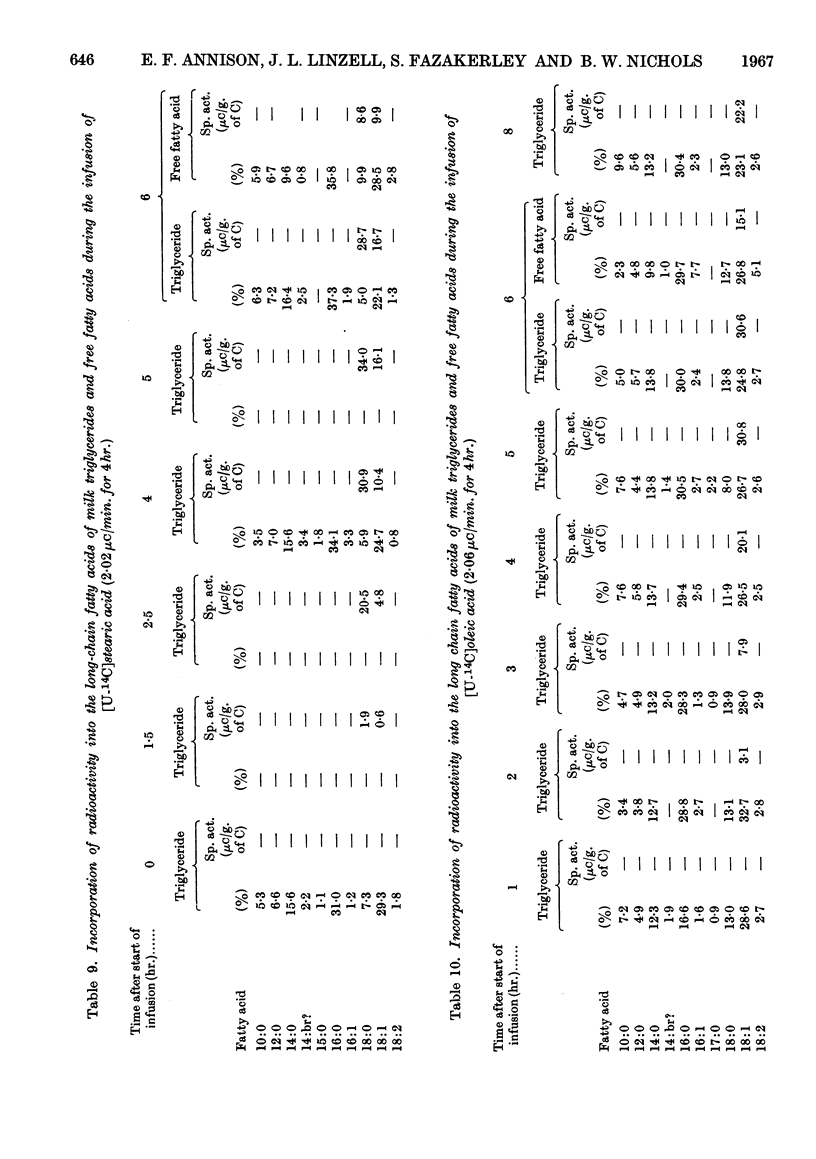
1. Measurements were made of milk yield, mammary blood flow and arteriovenous differences of each plasma lipid fraction, and their specific radioactivities, during the infusion of [U-14C]stearate, [U-14C]oleate, [U-14C]palmitate and [1-14C]acetate into fed lactating goats. 2. Entry rates of fatty acids into the circulation were 4·2mg./min./kg. body wt. for acetate, and 0·18, 0·28 and 0·42mg./min./kg. for stearate, oleate and palmitate respectively. Acetate accounted for 23% of the total carbon dioxide produced by the whole animal, and contributed to the oxidative metabolism of the mammary gland to about the same extent. Corresponding values for each of the long-chain acids were less than 1%. 3. There were no significant arteriovenous differences of phospholipids, sterols or sterol esters, and their fatty acid composition showed no net changes during passage through the mammary gland. 4. There were large arteriovenous differences of plasma triglycerides, and their fatty acid composition showed marked changes across the gland. The proportions of palmitate and stearate fell, and that of oleate increased. 5. Arteriovenous differences of plasma free fatty acids (FFA) were small and variable, but a large fall in the specific radioactivity of each of the long-chain acids examined indicated substantial uptake of plasma FFA, accompanied by roughly equivalent FFA release from mammary tissue. The uptake of FFA was confirmed by the extensive transfer of radioactivity into milk. The FFA of milk were similar in composition and radioactivity to the milk triglyceride fatty acids, and quite unlike plasma FFA. 6. The formation of large amounts of oleic acid (18–21 mg./min.) from stearic acid was demonstrated. 7. During the terminal stages of the [14C]acetate infusion, milk triglyceride fatty acids of chain length C4–C14 showed specific radioactivities that were 75–90% of that of blood acetate, and that of palmitate was roughly one-quarter of this value. Oleate and stearate were unlabelled. 8. The results confirmed that milk fatty acids of chain length C4–C14 arise largely from blood acetate, and palmitate is derived partly from acetate and partly from plasma triglyceride, the latter fraction being almost the sole precursor of oleate and stearate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., LINZELL J. L. THE OXIDATION AND UTILIZATION OF GLUCOSE AND ACETATE BY THE MAMMARY GLAND OF THE GOAT IN RELATION TO THEIR OVER-ALL METABOLISM AND MILK FORMATION. J Physiol. 1964 Dec;175:372–385. doi: 10.1113/jphysiol.1964.sp007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY J. M., BARTLEY W., LINZELL J. L., ROBINSON D. S. THE UPTAKE FROM THE BLOOD OF TRIGLYCERIDE FATTY ACIDS OF CHYLOMICRA AND LOW-DENSITY LIPOPROTEINS BY THE MAMMARY GLAND OF THE GOAT. Biochem J. 1963 Oct;89:6–11. doi: 10.1042/bj0890006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood J. H. The absorption of milk precursors by the mammary gland: The relation of phosphorus to the fat metabolism of lactation. Biochem J. 1934;28(4):1346–1354. doi: 10.1042/bj0281346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON L. A., WADSTROM L. B. Determination of glycerides in blood serum. Clin Chim Acta. 1959 Mar;4(2):197–205. doi: 10.1016/0009-8981(59)90130-5. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., JAMES A. T., WEBB J. P., RIZACK M. A., STURMAN M. F. The fatty acid patterns of plasma lipids during alimentary lipemia. J Clin Invest. 1959 Sep;38:1544–1554. doi: 10.1172/JCI103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GARTON G. A. THE COMPOSITION AND BIOSYNTHESIS OF MILK LIPIDS. J Lipid Res. 1963 Jul;4:237–254. [PubMed] [Google Scholar]
- GLASCOCK R. F., DUNCOMBE W. G., REINIUS L. R. Studies on the origin of milk fat. 2. The secretion of dietary long-chain fatty acids in milk fat by ruminants. Biochem J. 1956 Apr;62(4):535–541. doi: 10.1042/bj0620535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- HITCHCOCK C., JAMES A. T. OXIDATION OF UNSATURATED FATTY ACIDS BY LEAF TISSUE. J Lipid Res. 1964 Oct;5:593–599. [PubMed] [Google Scholar]
- KRONFELD D. S. PLASMA NON-ESTERIFIED FATTY ACID CONCENTRATIONS IN THE DAIRY COW: RESPONSES TO NUTRITIONAL AND HORMONAL STIMULI, AND SIGNIFICANCE IN KETOSIS. Vet Rec. 1965 Jan 9;77:30–35. [PubMed] [Google Scholar]
- LAURYSSENS M., VERBEKE R., PEETERS G., GARTON G. A., LOUGH A. K., DUNCAN W. R. [Metabolism of stearate-1-C14 in the bovine mammary gland]. Arch Int Physiol Biochim. 1960 May;68:511–512. [PubMed] [Google Scholar]
- LINZELL J. L. Mammary-gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. J Physiol. 1960 Oct;153:492–509. doi: 10.1113/jphysiol.1960.sp006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZELL J. L. Valvular incompetence in the venous drainage of the udder. J Physiol. 1960 Oct;153:481–491. doi: 10.1113/jphysiol.1960.sp006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. Measurement of venous flow by continuous thermodilution and its application to measurement of mammary blood flow in the goat. Circ Res. 1966 Jun;18(6):745–754. doi: 10.1161/01.res.18.6.745. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Fatty acid mobilization--mechanisms of regulation and metabolic consequences. Biochem Soc Symp. 1963;24:111–143. [PubMed] [Google Scholar]


