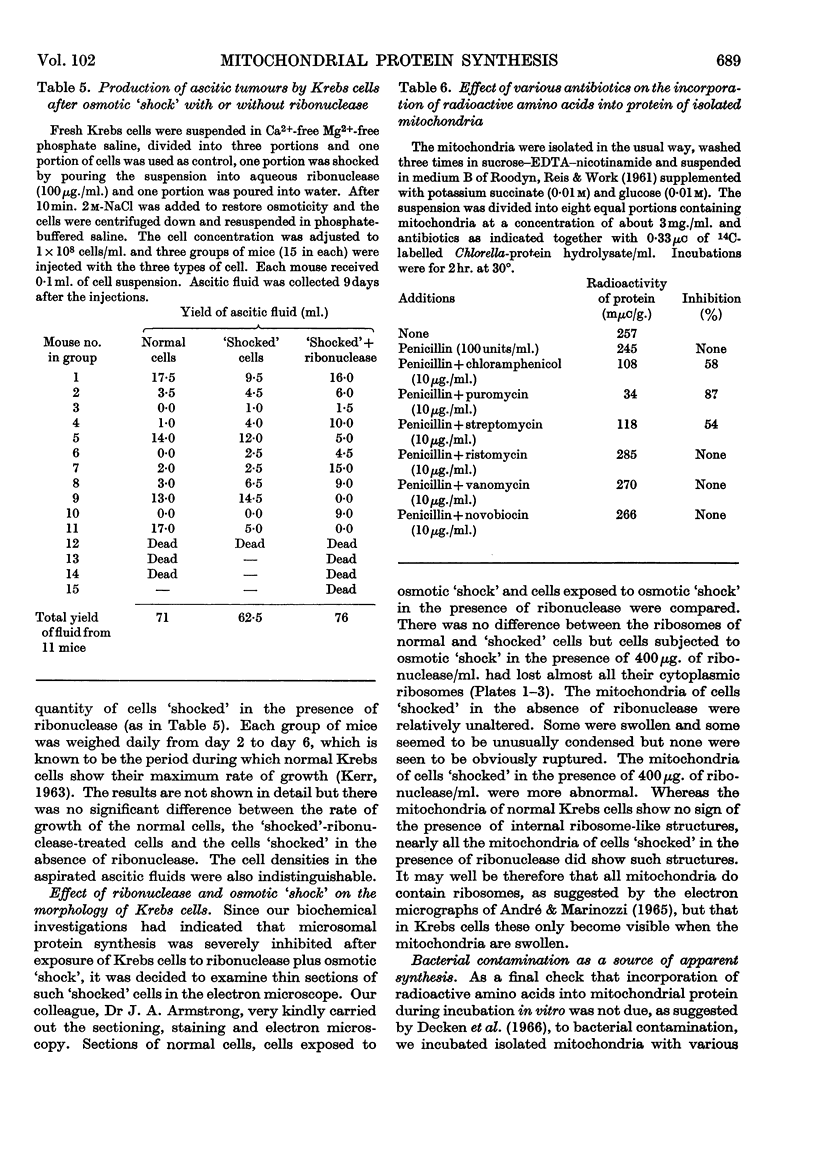
Abstract
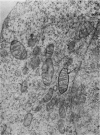
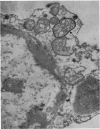
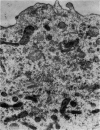
At 22° in Earle's medium, Krebs cells synthesize proteins. After a brief `pulse' with [14C]valine followed by a `chase' of [12C]valine the radioactivity appears first in microsomes and is transferred after `chase' to the cell sap. Kinetics of labelling of the mitochondrial protein are different from that of either microsomal or cell-sap protein. When Krebs cells in buffer are mixed with ribonuclease in water the nuclease penetrates the cell membrane. The ribonuclease-treated cells are still viable but have lost most of their cytoplasmic ribosomes (electron micrograph). Such cells still synthesize mitochondrial protein at near normal rate but synthesis of microsomal protein is severely inhibited. The results indicate that some mitochondrial proteins are synthesized independently of the microsome–cell-sap system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FREEMAN K. B. PROTEIN SYNTHESIS IN MITOCHONDRIA. 4. PREPARATION AND PROPERTIES OF MITOCHONDRIA FROM KREBS II MOUSE ASCITES-TUMOUR CELLS. Biochem J. 1965 Feb;94:494–501. doi: 10.1042/bj0940494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., KELLER E. B., GROSS J., ZAMECNIK P. C. Studies on cytoplasmic ribonucleoprotein particles from the liver of the rat. J Biol Chem. 1955 Nov;217(1):111–123. [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLEAN J. R., COHN G. L., BRANDT I. K., SIMPSON M. V. Incorporation of labeled amino acids into the protein of muscle and liver mitochondria. J Biol Chem. 1958 Sep;233(3):657–663. [PubMed] [Google Scholar]
- ROODYN D. B. Protein synthesis in mitochondria. 3. The controlled disruption and subfractionation of mitochondria labelled in vitro with radioactive valine. Biochem J. 1962 Oct;85:177–189. doi: 10.1042/bj0850177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMKIN J. L., WORK T. S. Incorporation of radioactive amino acids into proteins of the microsome fraction of guinea-pig liver in a cell-free system. Biochem J. 1957 Dec;67(4):617–624. doi: 10.1042/bj0670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., POSTGATE J. R. PENETRATION OF SUBSTANCES INTO COLD-SHOCKED BACTERIA. J Gen Microbiol. 1964 Sep;36:393–403. doi: 10.1099/00221287-36-3-393. [DOI] [PubMed] [Google Scholar]
- ZAMECNIK P. C., KELLER E. B. Relation between phosphate energy donors and incorporation of labeled amino acids into proteins. J Biol Chem. 1954 Jul;209(1):337–354. [PubMed] [Google Scholar]