Abstract
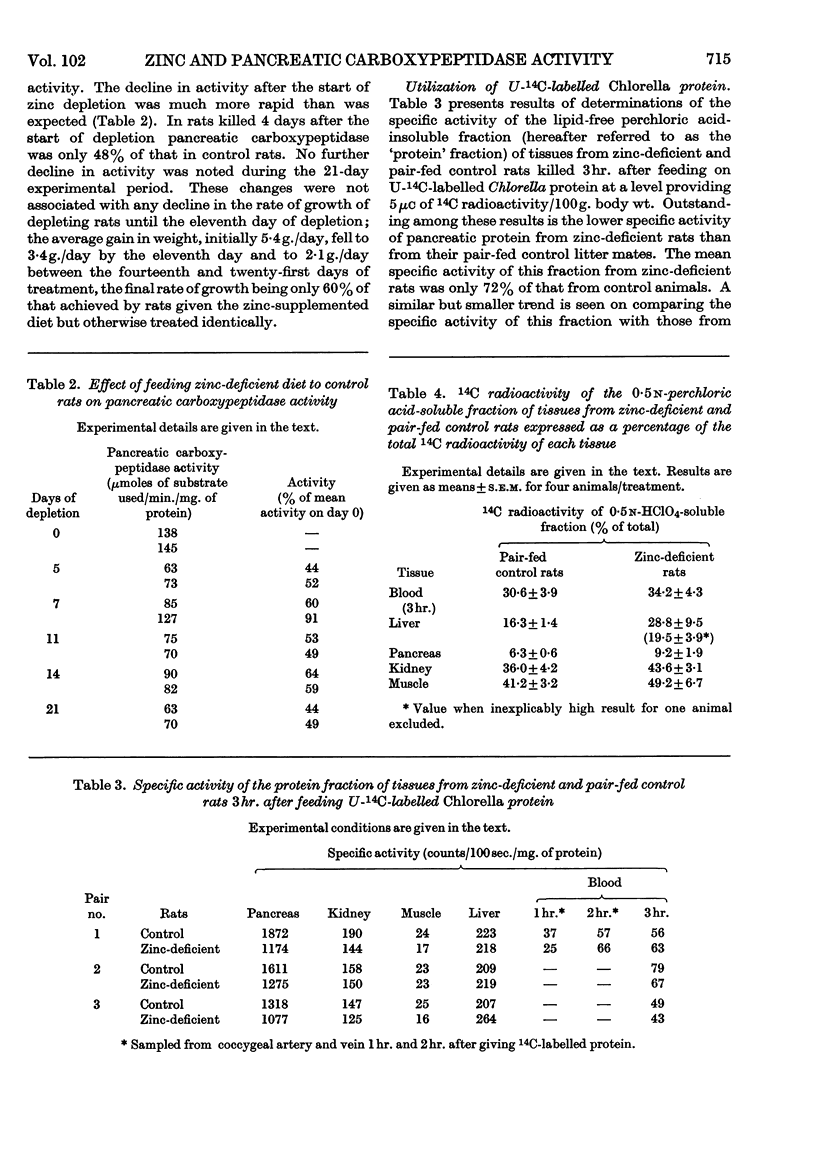
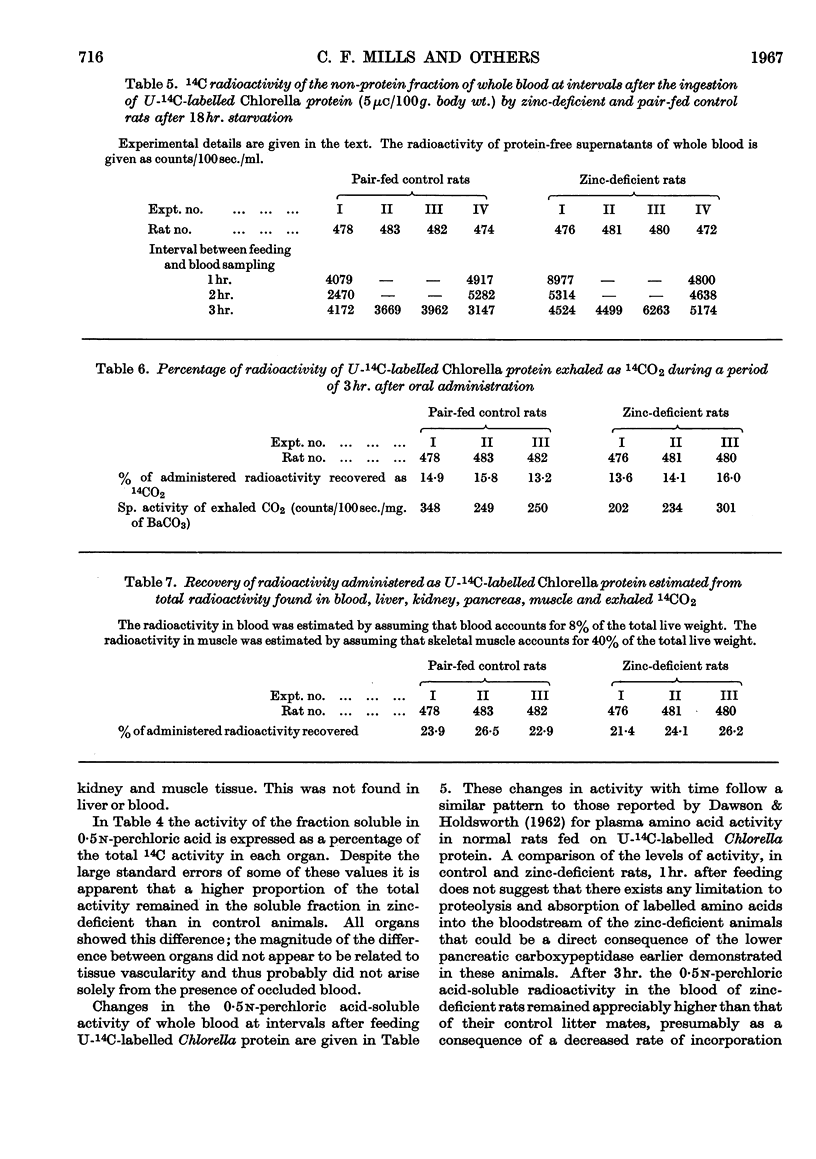
1. Proteolytic enzyme activities were examined in the pancreas of zinc-deficient and control rats. 2. No change was detected in trypsin-plus-chymotrypsin activity. 3. Carboxypeptidase activity was appreciably lowered in zinc deficiency and returned rapidly to normal on zinc therapy. 4. In experiments in which U-14C-labelled Chlorella protein was fed no evidence was obtained which suggested that the reduction in carboxypeptidase activity had limited the rate of protein digestion or absorption. 5. The specific activity of pancreatic protein synthesized during these experiments was appreciably lower in zinc-deficient than in control rats. 6. A higher proportion of the total activity present, in each organ examined, was in the non-protein fraction in zinc-deficient rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases. J Biol Chem. 1960 Feb;235:390–395. [PubMed] [Google Scholar]
- HOWARD F., YUDKIN J. EFFECT OF DIETARY CHANGE UPON THE AMYLASE AND TRYPSIN ACTIVITIES OF THE RAT PANCREAS. Br J Nutr. 1963;17:281–294. doi: 10.1079/bjn19630031. [DOI] [PubMed] [Google Scholar]
- Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940 Sep;34(8-9):1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGG S. P., SEARS L. Zinc sulphate treatment of parakeratosis in cattle. Nature. 1960 Jun 25;186:1061–1062. doi: 10.1038/1861061a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER J. K., MILLER W. J. Experimental zinc deficiency and recovery of calves. J Nutr. 1962 Apr;76:467–474. doi: 10.1093/jn/76.4.467. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- OTT E. A., SMITH W. H., STOB M., BEESON W. M. ZINC DEFICIENCY SYNDROME IN THE YOUNG LAMB. J Nutr. 1964 Jan;82:41–50. doi: 10.1093/jn/82.1.41. [DOI] [PubMed] [Google Scholar]
- PRASAD A. S., MIALE A., Jr, FARID Z., SANDSTEAD H. H., SCHULERT A. R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963 Apr;61:537–549. [PubMed] [Google Scholar]
- RAVIN H. A., SELIGMAN A. M. The colorimetric estimation of carboxypeptidase activity. J Biol Chem. 1951 May;190(1):391–402. [PubMed] [Google Scholar]
- TUCKER H. F., SALMON W. D. Parakeratosis or zinc deficiency disease in the pig. Proc Soc Exp Biol Med. 1955 Apr;88(4):613–616. doi: 10.3181/00379727-88-21670. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., NEURATH H. Carboxypeptidase, a zinc metalloenzyme. J Biol Chem. 1955 Nov;217(1):253–261. [PubMed] [Google Scholar]
- VALLEE B. L. The "active catalytic site," an approach through metalloenzymes. Fed Proc. 1961 Sep;20(3):71–80. [PubMed] [Google Scholar]


