Abstract
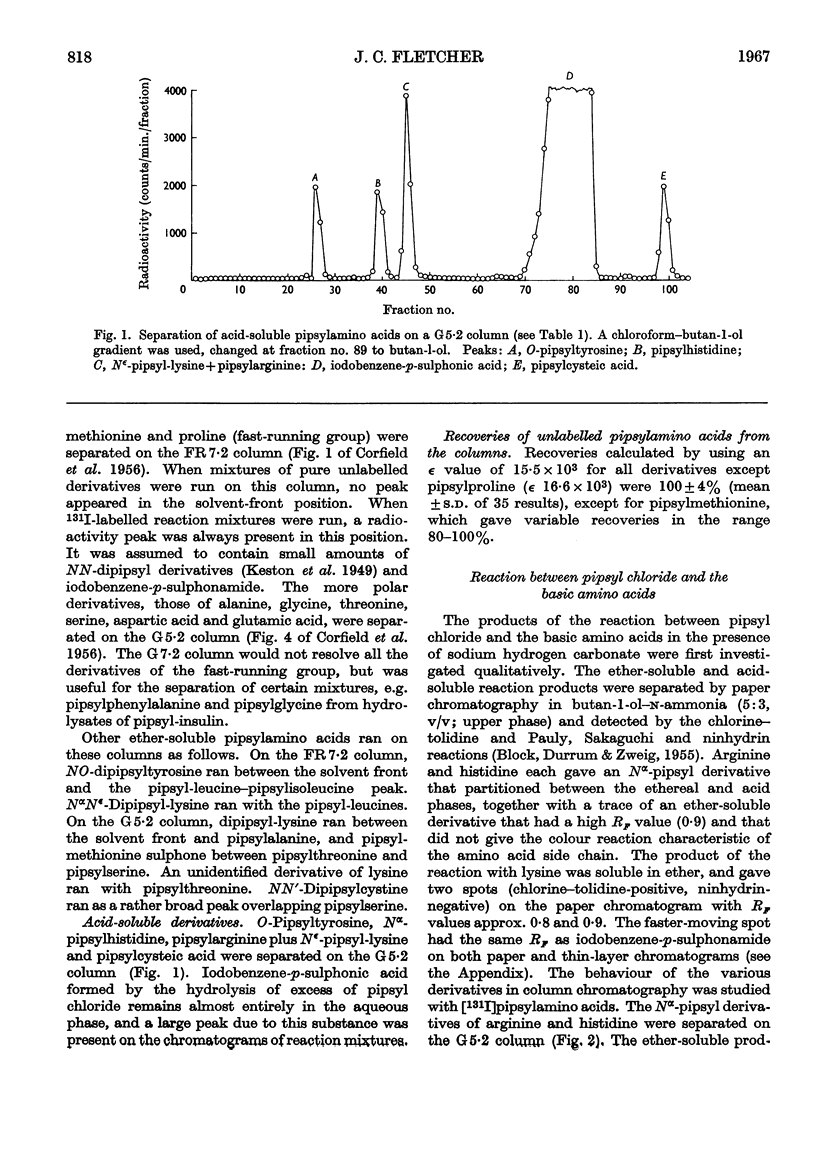
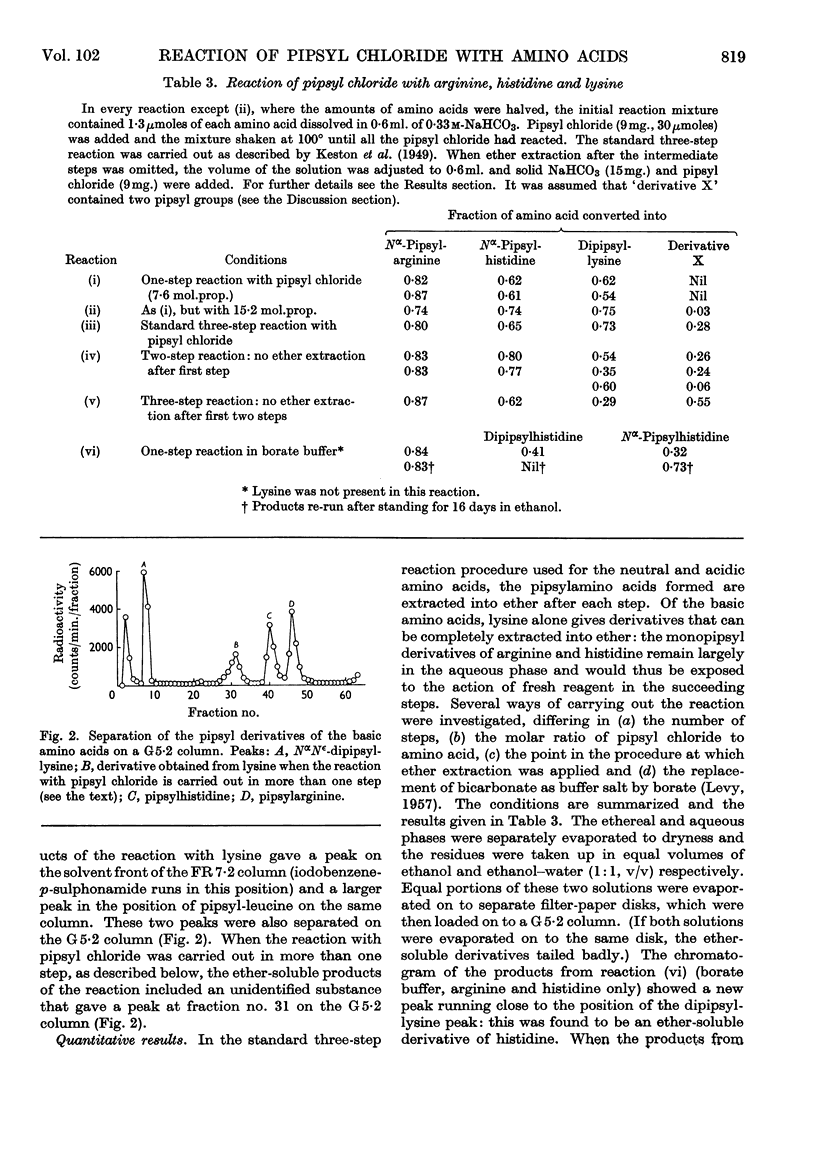
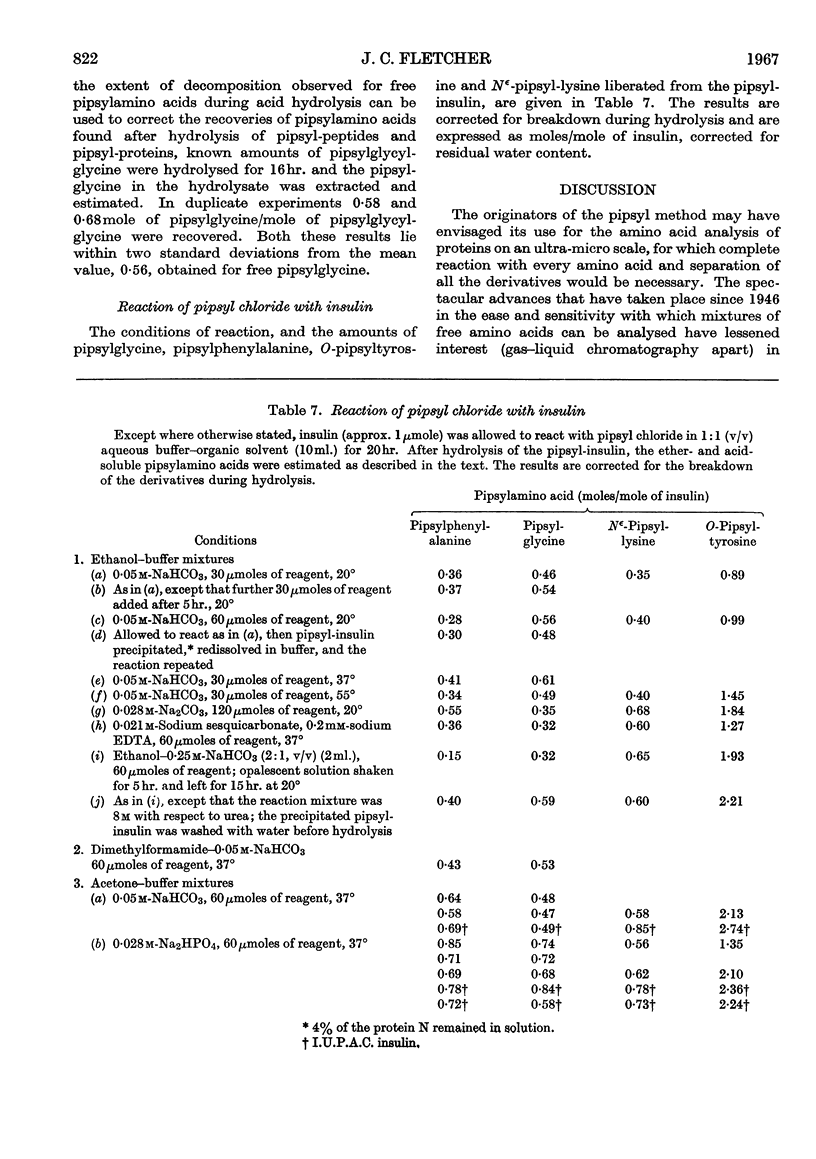
1. A system of separation using buffered Celite columns is described that enables the pipsyl derivatives of most of the common amino acids to be separated. 2. The reaction of pipsyl chloride with several amino acids not included in previous studies has been investigated. In particular, knowledge of the acid-soluble pipsyl derivatives of arginine, histidine, lysine, tyrosine and cysteic acid has been extended. 3. Reproducible factors have been obtained that enable corrections to be applied for the breakdown of pipsylamino acids on acid hydrolysis. 4. The reaction of pipsyl chloride with peptides has been studied under various conditions. 5. The extent of the reaction between pipsyl chloride and insulin depends on the nature of the solvent–buffer system, and under the best conditions so far found is about 75% complete. 6. In an Appendix, the separation of pipsylamino acids by thin-layer chromatography is described.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORFIELD M. C., DILWORTH S., FLETCHER J. C., GIBSON R. A machine for the automatic chromatography and assay of mixtures of radioactive substances. Int J Appl Radiat Isot. 1959 Feb;5(1):42–50. doi: 10.1016/0020-708x(59)90350-3. [DOI] [PubMed] [Google Scholar]
- CORFIELD M. C., ROBSON A., SKINNER B. The amino acid compositions of three fractions from oxidized wool. Biochem J. 1958 Feb;68(2):348–352. doi: 10.1042/bj0680348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield M. C., Fletcher J. C., Robson A. Amino acid sequences of peptides from a tryptic digest of a urea-soluble protein fraction (U.S.3) from oxidized wool. Biochem J. 1967 Mar;102(3):801–814. doi: 10.1042/bj1020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R. L., SAROFF H. A. A physiologically active guanidinated derivative of insulin. J Biol Chem. 1957 Sep;228(1):295–304. [PubMed] [Google Scholar]
- KALLOS J., RIZOK D. HEAVY ATOM LABELLING OF THE SERINE OF THE ACTIVE CENTRE OF CHYMOTRYPSIN: PIPSYL-CHYMOTRYPSIN. J Mol Biol. 1964 Jul;9:255–259. doi: 10.1016/s0022-2836(64)80107-8. [DOI] [PubMed] [Google Scholar]
- LEVY M., SLOBODIAN E. Sequences of amino acid residues in silk fibroin. J Biol Chem. 1952 Dec;199(2):563–572. [PubMed] [Google Scholar]
- MATHESON N. A. THE ISOLATION OF AMINO ACIDS FROM MIXTURES AS THEIR N-2,4-DINITROPHENYL DERIVATIVES, METHIONINE, LEUCINE, PHENYLALANINE, ORNITHINE, LYSINE AND TYROSINE. Biochem J. 1965 Mar;94:513–517. doi: 10.1042/bj0940513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neadle D. J., Pollitt R. J. The formation of 1-dimethylaminonaphthalene-5-sulphonamide during the preparation of 1-dimethylaminonaphthalene-5-sulphonylamino acids. Biochem J. 1965 Nov;97(2):607–608. doi: 10.1042/bj0970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oratz M., Burks A. L., Rothschild M. A. The determination of free epsilon-amino groups in proteins. Biochim Biophys Acta. 1966 Jan 25;115(1):88–94. doi: 10.1016/0304-4165(66)90052-3. [DOI] [PubMed] [Google Scholar]
- SLOBODIAN E., LEVY M. The sequence glycylalanylglycine in silk fibroin. J Biol Chem. 1953 Mar;201(1):371–375. [PubMed] [Google Scholar]
- UDENFRIEND S., VELICK S. F. The isotope derivative method of protein amino end-group analysis. J Biol Chem. 1951 Jun;190(2):733–740. [PubMed] [Google Scholar]
- VELICK S. F., UDENFRIEND S. Isotope derivative analysis for proline, valine, methionine, and phenylalanine. J Biol Chem. 1951 Jun;190(2):721–731. [PubMed] [Google Scholar]
- VELICK S. F., UDENFRIEND S. The amino end-group and the amino acid composition of salmine. J Biol Chem. 1951 Jul;191(1):233–238. [PubMed] [Google Scholar]
- VELICK S. F., UDENFRIEND S. The composition and amino acid end-groups of glyceraldehyde-3-phosphate dehydrogenases. J Biol Chem. 1953 Aug;203(2):575–582. [PubMed] [Google Scholar]


