Abstract
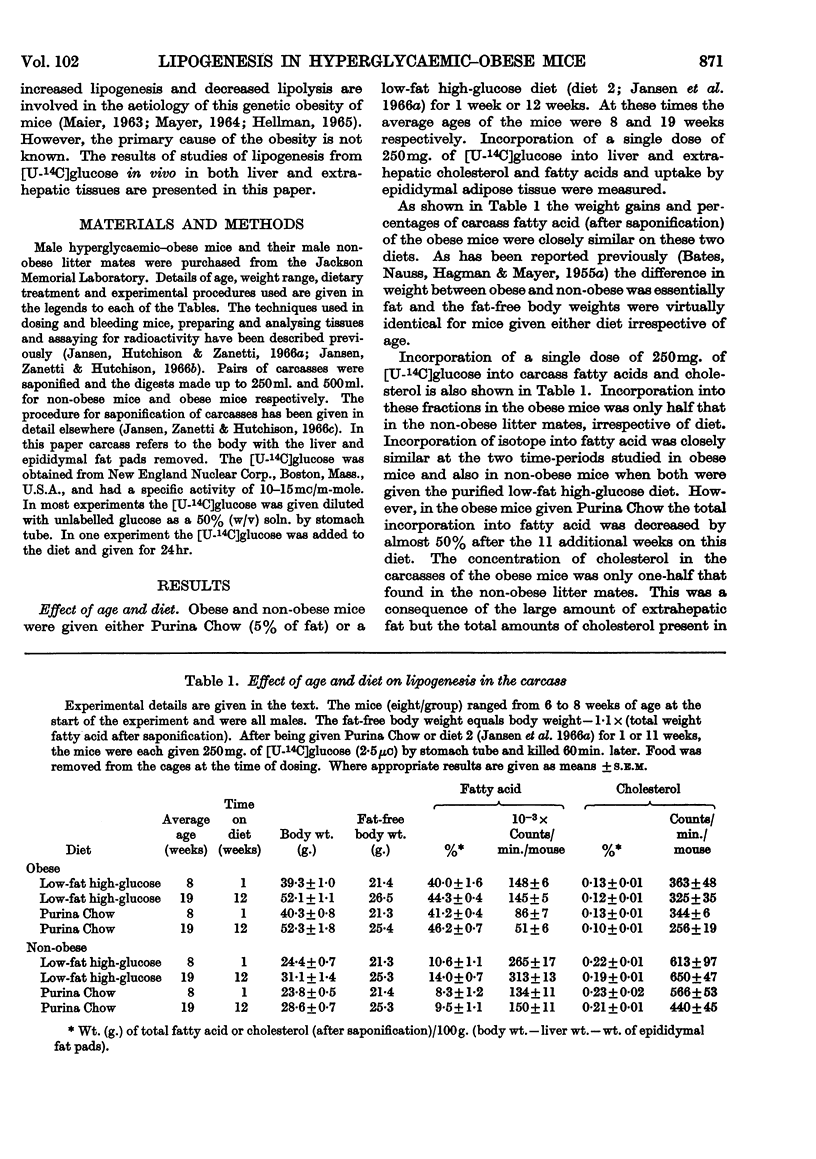
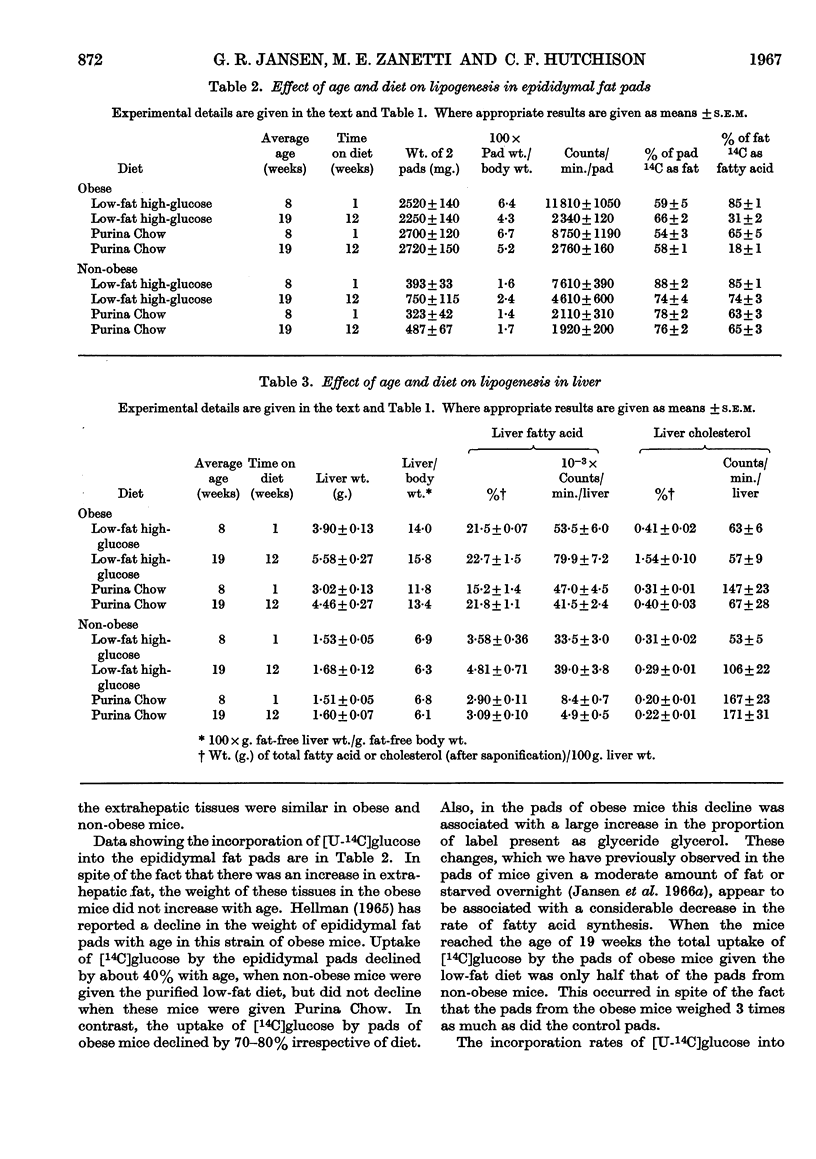
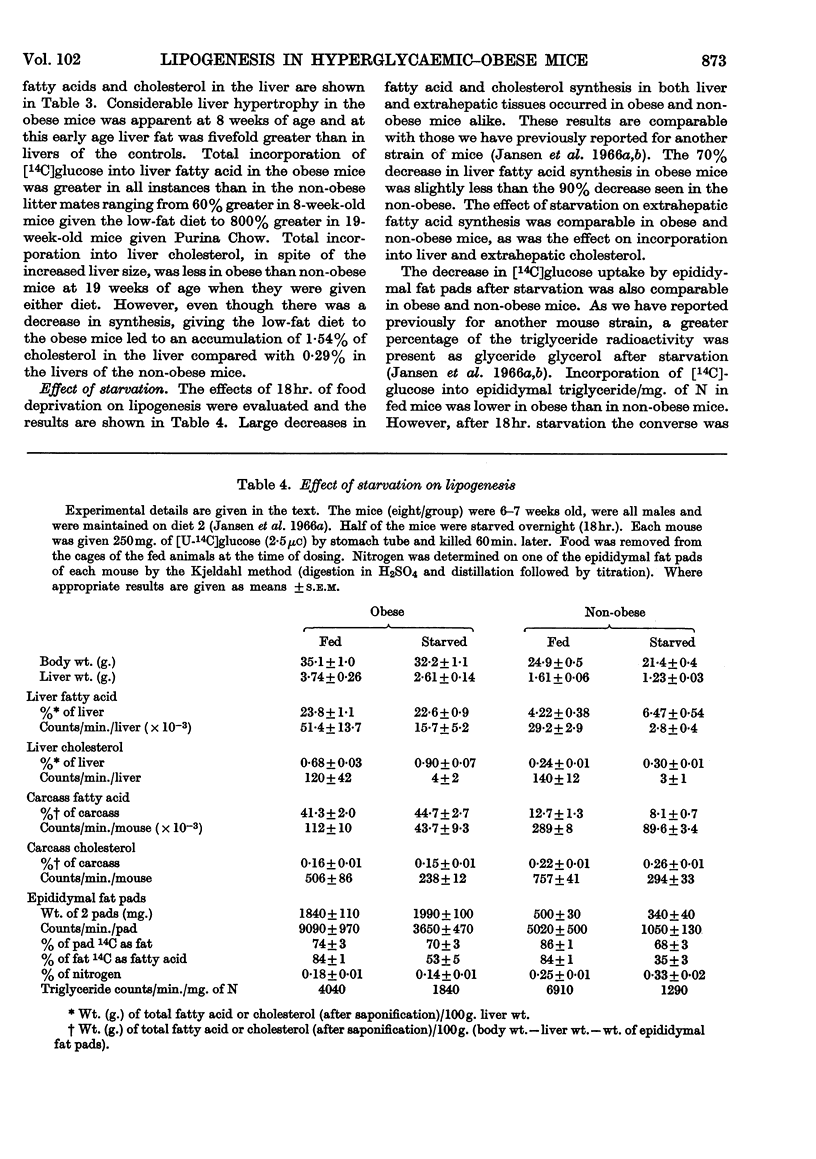
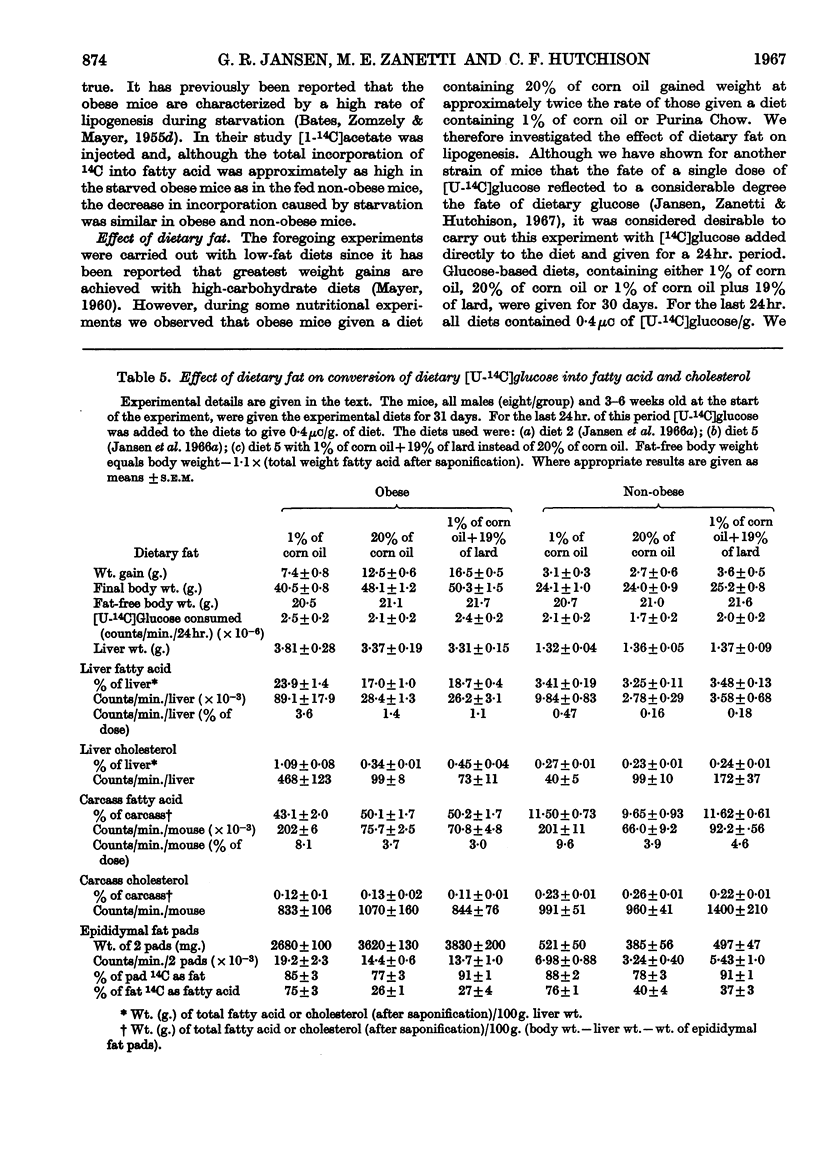
1. Lipogenesis has been studied in intact genetically obese mice by measuring the incorporation of a single oral dose of 250mg. of [U-14C]glucose into fatty acid and cholesterol in the liver and extrahepatic tissues. Studies were also carried out with [U-14C]glucose added to the diet and fed for 24hr. With either method of isotope administration, the conversion of [U-14C]glucose into fatty acid was greatly elevated in the livers of the obese mice. In contrast, conversion of the single dose of [14C]glucose into fatty acid in extrahepatic tissues of obese mice was only half that occurring in the non-obese litter mates. When [14C]glucose was given in the diet for 24hr. the total accumulation of labelled fatty acid in extrahepatic tissues of obese mice was slightly less than in the non-obese. Uptake of labelled glucose and conversion into fatty acid in adipose tissue of the obese mice decreased with age. 2. Conversion of the single dose of [14C]glucose into liver cholesterol was comparable in obese and non-obese mice fed on a purified low-fat diet. However, obese mice given this diet for 12 weeks accumulated 1·54% of cholesterol in the liver compared with 0·29% in the non-obese litter mates. This accumulation apparently resulted from a decrease in removal of cholesterol from the liver, rather than an increased synthesis. 3. Conversion of the single dose of [14C]glucose into extrahepatic fatty acid was decreased by 18hr. starvation proportionally as much in obese as in non-obese mice. The decrease in liver fatty acid synthesis caused by starvation also was considerable in obese mice, although somewhat less marked than in the non-obese. 4. The metabolic derangements in the liver could be more fundamental to the development of the obesity than the changes seen in extrahepatic tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES M. W., MAYER J., NAUSS S. F. Fat metabolism in three forms of experimental obesity; acetate incorporation. Am J Physiol. 1955 Feb;180(2):304–308. doi: 10.1152/ajplegacy.1955.180.2.304. [DOI] [PubMed] [Google Scholar]
- BATES M. W., MAYER J., NAUSS S. F. Fat metabolism in three forms of experimental obesity; fatty acid turnover. Am J Physiol. 1955 Feb;180(2):309–312. doi: 10.1152/ajplegacy.1955.180.2.309. [DOI] [PubMed] [Google Scholar]
- BATES M. W., NAUSS S. F., HAGMAN N. C., MAYER J. Fat metabolism in three forms of experimental obesity; body composition. Am J Physiol. 1955 Feb;180(2):301–303. doi: 10.1152/ajplegacy.1955.180.2.301. [DOI] [PubMed] [Google Scholar]
- CHRISTOPHE J., JEANRENAUD B., MAYER J., RENOLD A. E. Metabolism in vitro of adipose tissue in obese-hyperglycemic and goldthioglucose-treated mice. I. Metabolism of glucose. J Biol Chem. 1961 Mar;236:642–647. [PubMed] [Google Scholar]
- CHRISTOPHE J., JEANRENAUD B., MAYER J., RENOLD A. E. Metabolism in vitro of adipose tissue in obese-hyperglycemic and goldthioglucose-treated mice. II. Metabolism of pyruvate and acetate. J Biol Chem. 1961 Mar;236:648–652. [PubMed] [Google Scholar]
- HAUSBERGER F. X. Behavior of transplanted adipose tissue of hereditarily obese mice. Anat Rec. 1959 Oct;135:109–113. doi: 10.1002/ar.1091350205. [DOI] [PubMed] [Google Scholar]
- HELLMAN B., LARSSON S., WESTMAN S. Acetate metabolism in isolated epididymal adipost tissue from obese-hyperglycemic mice of different ages. Acta Physiol Scand. 1962 Oct;56:189–198. doi: 10.1111/j.1748-1716.1962.tb02495.x. [DOI] [PubMed] [Google Scholar]
- HUGHES A. M., TOLBERT B. M. Oxidation of acetate, glucose, or glycine to carbon dioxide in mice exhibiting the hereditary obesity syndrome. J Biol Chem. 1958 Mar;231(1):339–345. [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- INGALLS A. M., DICKIE M. M., SNELL G. D. Obese, a new mutation in the house mouse. J Hered. 1950 Dec;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Jansen G. R., Hutchon C. F., Zanetti M. E. Studies on lipogenesis in vivo. Effect of dietary fat or starvation on conversion of [14]glucose into fat ad turnover of newly synthsized fat. Biochem J. 1966 May;99(2):323–332. doi: 10.1042/bj0990323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Stdies on lipogenesis in vivo. Effects of starvation andre-feeding, and studies on cholesterol synthesis. Biochem J. 1966 May;99(2):333–340. doi: 10.1042/bj0990333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Studies in lipogenesis in vivo: Fatty acid and cholesterol synthesis during starvation and re-feeding. Biochem J. 1966 Dec;101(3):811–818. doi: 10.1042/bj1010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Studies on lipogenesis in vivo: Comparison of cholesterol and fatty acid synthesis in rats and mice. Biochem J. 1967 Mar;102(3):864–869. doi: 10.1042/bj1020864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE CLEAVAGE ENZYME IN LIVERS OF OBESE AND NONOBESE MICE. Science. 1964 May 22;144(3621):1027–1028. doi: 10.1126/science.144.3621.1027. [DOI] [PubMed] [Google Scholar]
- LOCHAYA S., HAMILTON J. C., MAYER J. Lipase and glycero-kinase activities in the adipose tissue of obese-hyperglycaemic mice. Nature. 1963 Jan 12;197:182–183. doi: 10.1038/197182a0. [DOI] [PubMed] [Google Scholar]
- MAYER J., HAGMAN N. C., MARSHALL N. B., STOOPS A. J. Fat metabolism in three forms of obesity. V. Hepatic lipogenesis in vitro. Am J Physiol. 1955 Jun;181(3):501–503. doi: 10.1152/ajplegacy.1955.181.3.501. [DOI] [PubMed] [Google Scholar]
- MAYER J., RUSSELL R. E., BATES M. W., DICKIE M. M. Metabolic, nutritional and endocrine studies of the hereditary obesity-diabetes syndrome of mice and mechanism of its development. Metabolism. 1953 Jan;2(1):9–21. [PubMed] [Google Scholar]
- Mayer J. Antagonism between alloxan and caffeine. Nature. 1966 May 7;210(5036):630–631. doi: 10.1038/210630a0. [DOI] [PubMed] [Google Scholar]
- NORBY J. G. EFFECTS OF GIVING A FAT-FREE DIET FOR UP TO 10 WEEKS ON THE MALE WEANLING RAT. Br J Nutr. 1965;19:209–224. doi: 10.1079/bjn19650021. [DOI] [PubMed] [Google Scholar]
- PARSON W., CRISPELL K. R. Studies of acetate metabolism in the hereditary obesity-diabetes syndrome of mice utilizing C14 acetate. Metabolism. 1955 May;4(3):227–230. [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- SHIGETA Y., SHREEVE W. W. FATTY ACID SYNTHESIS FROM GLUCOSE-I-H3 AND GLUCOSE-I-C14 IN OBESE-HYPERGLYCEMIC MICE. Am J Physiol. 1964 May;206:1085–1090. doi: 10.1152/ajplegacy.1964.206.5.1085. [DOI] [PubMed] [Google Scholar]
- TREBLE D. H., MAYER J. GLYCEROLKINASE ACTIVITY IN WHITE ADIPOSE TISSUE OF OBESE-HYPERGLYCAEMIC MICE. Nature. 1963 Oct 26;200:363–364. doi: 10.1038/200363a0. [DOI] [PubMed] [Google Scholar]


