Abstract
The impact of chronic prophylactic administration of trimethoprim-sulfamethoxazole (SXT) on the ecology and the antimicrobial susceptibilities of bloodstream pathogens in human immunodeficiency virus (HIV)-infected patients was studied using a retrospective chart review. Eighty-nine patients with advanced HIV infection developed 124 episodes of bacteremia with 156 pathogenic isolates. Staphylococcus aureus and Enterobacteriaceae tended to be less common among patients receiving SXT. Isolates from patients receiving SXT were likelier (75%) to be resistant to 20 μg of SXT/ml than those from patients not receiving SXT (33%) (P < 0.001).
Human immunodeficiency virus (HIV)-infected patients are at increased risk for serious bacterial infections in addition to the broad array of infections more traditionally classified as “opportunistic” (1, 11, 15). Bacteremic episodes contribute significant morbidity and mortality in AIDS patients (1, 11, 18).
Trimethoprim-sulfamethoxazole (SXT), or cotrimoxazole, is widely used in HIV-infected patients who are at risk for the opportunistic infections Pneumocystis carinii pneumonia (PCP) and toxoplasmosis. An additional benefit of SXT use in HIV-infected patients (2, 4, 9; H. B. Mayer, D. N. Rose, S. Cohen, A. C. Gurtman, T. W. Cheung, and S. Szabo, Letter, AIDS, 7:1687-1689, 1993). and other immunocompromised patients (3, 8, 10) may be an overall reduction in bacterial infections. However, the selective pressure of chronic antibiotic use may alter the colonizing flora and susceptibility patterns of bacterial isolates (3, 4) should bacterial infections develop while patients are receiving chronic SXT therapy.
(A portion of the data was previously presented [D. A. Wininger and R. J. Fass, Abstr. 36th Ann. Meet. Infect. Dis. Soc. Am., abstr. 420 Fr, 1998].)
Medical records of patients (excluding prisoners) hospitalized at the Ohio State University Medical Center between June 23, 1991, and June 23, 1997, were searched by cross-referencing diagnostic codes for HIV and terms for bacteremia and septicemia. In review of the records, the 1988 Centers for Disease Control definition of bloodstream infection was used (7). Bloodstream infections with mycobacteria, fungi, and viruses were not included.
Data abstracted from the medical records of patients with bacteremia included demographics (age, sex, and race), HIV acquisition risk factors, past and present AIDS-defining conditions, use of intravascular access devices, antimicrobial use within 2 weeks preceding bacteremia, antiretroviral therapy history, proximate CD4+-lymphocyte count, white blood cell count (WBCC), absolute neutrophil count (ANC), positive blood culture reports with susceptibilities, and survival to discharge. SXT use was defined as having received the agent on a scheduled basis within the 2 weeks prior to the first positive blood culture for the episode. Portals of entry were determined by the primary investigator based on clinical diagnoses, laboratory and radiographic studies, and positive cultures from sites other than blood. Relapses, defined as isolation of the same bacterial species with the same or similar antibiogram within 3 months of the prior episode, were not included.
MICs were determined by a microdilution method (14). Based on the observed frequency distribution of SXT MICs for the study strains, a MIC of ≤20 μg/ml was considered to represent susceptibility and a MIC of ≥40 μg/ml was considered to represent resistance. This breakpoint best dichotomized these strains. It was 1 dilution lower than the one recommended by the NCCLS, which, if used, would not have altered conclusions.
Statistical analyses were performed using Stata version 6.0. To determine the statistical significance of continuous variables, the Wilcoxon rank sum test was used. To determine the statistical significance of categorical variables, Pearson's χ2 test and Fisher's exact test were used.
Of the 89 patients, 90% were male and 10% were female. The mean age was 37± 6.6 (standard deviation [SD]) years. Of the patients, 72% were white, 27% were black, and one was non-black Hispanic. The risk factors for HIV acquisition are shown in Table 1.
TABLE 1.
HIV risk factors among 89 patients with bacteremiaa
| Risk factor | No. (%) |
|---|---|
| Sex between men | 43 (48) |
| Intravenous drug use | 15 (17) |
| Heterosexual contact | 9 (10) |
| Receipt of blood products | 7 (8) |
| Sex with intravenous drug user | 5 (6) |
| Other | 2 (2) |
| Unknown | 16 (18) |
Some patients had more than one risk factor.
Eighty-seven (98%) of the 89 patients had advanced HIV disease based on CD4+-lymphocyte counts of <200 cells/μl (97% of 67 with recorded values), AIDS-defining illnesses (88%) (Table 2), and/or the receipt of prophylactic antibiotics for PCP (79%). Forty-seven (53%) were receiving SXT at the time of their bacteremias. There were no significant differences in CD4+-lymphocyte counts, WBCCs, or ANCs among the patients who received SXT prophylaxis and those who did not. The mean WBCC was 4,290 ± 3,310 cells/μl (n = 47) versus 4,440 ± 3,860/μl (n = 42) (P = 0.831); the mean ANC was 3,198 ± 2,809 cells/μl (n = 45) versus 3,712 ± 3,658 (n = 42) (P = 0.922); the mean CD4+-lymphocyte count was 40 ± 50 cells/μl (n = 36) versus 51 ± 78/μl (n = 31) (P = 0.375) (all by Wilcoxon rank sum).
TABLE 2.
AIDS-defining illnesses among 89 patients with bacteremiaa
| AIDS-defining illness | No. (%) |
|---|---|
| Cytomegalovirus | 69 (56) |
| PCP | 52 (42) |
| Mycobacterium avium complex | 36 (29) |
| Esophageal candidiasis | 27 (22) |
| Cryptococcosis | 17 (14) |
| Lymphoma | 12 (10) |
| Kaposi's sarcoma | 11 (9) |
| Cryptosporidiosis | 11 (9) |
| Histoplasmosis | 5 (4) |
| Toxoplasmosis | 2 (2) |
Some patients had more than one AIDS-defining illness.
During the years of this study, HIV viral load measurements were not routinely performed. Although 71% had received some form of antiretroviral therapy, only 7% had received protease inhibitors. These bacteremias occurred almost exclusively in HIV-infected patients who were receiving inadequate antiretroviral regimens by present standards.
The most common identifiable portal of entry for bacteremia was an intravenous catheter (IVC) used to treat opportunistic infection, primarily cytomegalovirus retinitis. IVC-related bacteremias occurred more commonly in patients on SXT, while bacteremias related to pulmonary infections occurred more commonly in patients not receiving SXT (P = 0.172, χ2 test).
There were 124 bacteremia episodes among the 89 HIV-infected patients; 20 patients had multiple individual episodes. Table 3 categorizes the 156 blood isolates from the 124 episodes of bacteremia; 22 episodes were polymicrobial. Table 4 shows that the etiology of bacteremias was associated with the portal of entry (P < 0.001). Bacteremias with staphylococci were associated with IVC infections, while those with Streptococcus pneumoniae and Pseudomonas aeruginosa were usually associated with pulmonary infections.
TABLE 3.
Blood isolates (n = 156) from 124 episodes of bacteremiaa
| Organism | No. (%) |
|---|---|
| Coagulase-negative staphylococci | 50 (32) |
| P. aeruginosa | 19 (12) |
| S. aureus | 15 (10) |
| Escherichia coli | 11 (7) |
| Enterococcus species | 11 (7) |
| Klebsiella and Enterobacter species | 10 (6) |
| S. pneumoniae | 10 (6) |
| Other Streptococcus species | 7 (4) |
| Corynebacterium species | 4 (3) |
| Acinetobacter species | 4 (3) |
| Stenotrophomonas maltophilia | 2 (1) |
| Other | 13 (8) |
Twenty patients had multiple individual episodes of bacteremia, and 22 episodes were polymicrobial.
TABLE 4.
Relationship of organism and portal of entry
| Organism | Total no. of patientsa | Portal of entryb
|
P (Fisher's exact test)c | ||
|---|---|---|---|---|---|
| IVC | Pulmonary | Other | |||
| No. (%) | No. (%) | No. (%) | |||
| S. aureus | 14 | 5 (50) | 1 (10) | 4 (40) | 0.102 |
| Coagulase-negative staphylococci | 30 | 28 (97) | 1 (3) | 0 (0) | <0.001 |
| S. pneumoniae | 10 | 1 (10) | 9 (90) | 0 (0) | <0.001 |
| Other Streptococcus species | 6 | 2 (67) | 0 (0) | 1 (33) | 0.412 |
| Enterococcus species | 9 | 4 (50) | 1 (13) | 3 (38) | 0.220 |
| Enterobacteriaceaed | 18 | 10 (67) | 2 (13) | 3 (20) | 0.381 |
| P. aeruginosa | 17 | 4 (31) | 8 (62) | 1 (8) | 0.019 |
| Other | 18 | 13 (81) | 1 (6) | 2 (13) | 0.038 |
Includes the total number of patients that ever had the organism isolated from a blood culture. This table does not take into consideration the number of times that the patients had a particular organism.
The portal of entry was unknown for 17 patients, accounting for the differences between totals and itemized categories for some organisms.
The overall relationship between organism and portal of entry by χ2 test (P < 0.001).
Includes E. coli, Klebsiella species, and Enterobacter species.
The frequencies of various blood isolates from patients receiving or not receiving SXT were compared. Those patients who were infected with Staphylococcus aureus or Enterobacteriaceae were likelier not to have received SXT (P = 0.163 and P = 0.185, respectively, χ2 test).
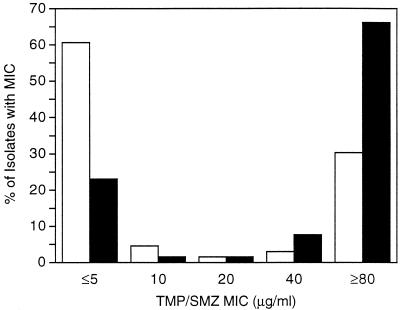
The SXT MICs for 131 of the 156 blood isolates were determined and are shown in Fig. 1;enterococci accounted for 11 of the 25 isolates not included. Isolates from patients receiving SXT were likelier to be resistant than those from patients not receiving SXT (P < 0.001), according to the Wilocoxon rank sum test for continuous variables or the χ2 test for dichotomous variables (susceptible, MIC ≤ 20 μg/ml; and resistant, MIC ≥ 40 μg/ml).
FIG 1.
<1> d0150 X: C S: ©MIC frequency distribution for blood isolates from patients receiving SXT (black bars; n = 65) and patients not receiving SXT (white bars; n = 66). TMP/SMZ, SXT.
The relationship of susceptibility to SXT and the organism, based on whether or not patients were receiving that drug, is shown in Table 5. For all individual species and groups of species tested, isolates from patients receiving prophylactic SXT were likelier to be resistant than those from patients not receiving SXT.
TABLE 5.
Relationship of organism susceptibility and SXT exposurea
| Organism | Results for those who:
|
P (Fisher's exact test) | ||||
|---|---|---|---|---|---|---|
| Received SXT
|
Did not receive SXT
|
|||||
| No. (%) (SXT MIC ≤ 20 μg/ml) | No. (%) (SXT MIC ≥ 40 μg/ml) | No. (%) (SXT MIC ≤ 20 μg/ml) | No. (%) (SXT MIC ≥ 40 μg/ml) | |||
| S. aureus | 2 (40) | 3 (60) | 10 (100) | 0 (0) | 0.022 | |
| Coagulase-negative staphylococci | 5 (19) | 22 (81) | 2 (43) | 12 (57) | 0.109 | |
| S. pneumoniae | 0 (0) | 3 (100) | 5 (83) | 1 (17) | 0.048 | |
| Other Streptococcus species | 0 (0) | 1 (100) | 4 (100) | 0 (100) | 0.200 | |
| Enterobacteriaceaeb | 2 (29) | 5 (71) | 12 (86) | 2 (14) | 0.017 | |
| P. aeruginosa | 0 (0) | 11 (100) | 0 (0) | 7 (100) | NAc | |
| Other | 7 (78) | 2 (22) | 5 (83) | 1 (17) | 1.000 | |
Includes all 131 isolates that were tested irrespective of the number of times isolated from individual patients.
Includes E. coli, Klebsiella species, and Enterobacter species.
NA, not applicable.
Twenty-four (27%) of the 89 bacteremic patients died during hospitalization; half had received SXT, while half had not (P = 0.92, χ2). As others have observed (16), SXT exposure in HIV-infected patients had no demonstrable impact on mortality from bacteremia. Thirteen deaths were attributed to bacteremia, while 11 were attributed to coexisting conditions. CD4+-lymphocyte count did not predict mortality; for those who lived, the mean was 45 ± 69 (SD) cells/μl compared to 44 ± 48 (SD) cells/μl for those who died (P = 0.449, Wilcoxon rank sum). There was also no relationship between the ANC at the time of bacteremia and mortality for the 124 episodes of bacteremia.
Soon after the introduction of antibacterial agents (before HIV infection had been observed), it was noted that antimicrobial therapy changed the ecology of bacterial infections (5) and the susceptibilities of common bacterial pathogens to prescribed antimicrobials (6). In a modern study of 1,717 community-acquired bacteremias in Denmark (17), antibiotic use within the 3 months prior to the bacteremia was strongly associated with homologous resistance to ampicillin (odds ratio [OR], 2.8; 95% confidence interval [CI] 1.8 to 4.5), sulfonamides (OR, 3.5; 95% CI, 2.1 to 5.9), and trimethoprim (OR, 14.3; 95% CI, 6.3 to 32.4).
Our data demonstrating the emergence of infections with SXT-resistant organisms in patients receiving SXT prophylaxis should be considered in the context of the positive impact that this antimicrobial has had for patients with AIDS. In addition to the widespread, successful use of SXT to prevent PCP and toxoplasmosis among patients with advanced HIV infection, most studies (2, 4, 9, 10; Mayer et al., letter; M. Tumbarello, E. Tacconelli, R. Cauda, and L. Ortona, Letter, AIDS, 11:1070-1071, 1997) have demonstrated a reduction in the frequency of bacteremia, respiratory tract infections, and other serious bacterial infections in those patients.
Few studies of HIV-associated bacteremia have addressed the impact of antimicrobial prophylaxis on the ecology of bacterial infections and antimicrobial resistance (4, 13; Tumbarello et al., letter). In some studies there was a reduced frequency of salmonella and S. pneumoniae bacteremias in those patients on SXT prophylaxis. An impact of SXT exposure on eventual SXT resistance was noted (4).
The association of SXT resistance with SXT use has been demonstrated in a serial cross-sectional study of SXT resistance at San Francisco General Hospital (12). The frequency of resistance among bacterial isolates from wards populated with HIV-infected patients rose more than the frequencies among isolates from comparative wards during the years of rising SXT prophylaxis in HIV-infected patients. The association of SXT resistance with SXT use was impressive but was only presumptive, however, because data on individual patients were not available.
The present study demonstrated an impact of prophylactic SXT on both the ecology of bacteremias in HIV-infected patients and the susceptibilities of their blood isolates to that drug combination. Although the numbers of isolates for each species were too small to achieve statistical significance, organisms that are typically SXT susceptible (S. aureus and Enterobacteriaceae) were relatively less common among patients who developed bacteremia while receiving SXT. The impact of SXT use on the susceptibilities of the blood isolates was clear, with those from patients receiving SXT being more than twice as likely to be SXT resistant than those from patients not receiving SXT. A limitation of this retrospective study is that it captures only those patients with episodes of bacteremia and does not describe the impact of SXT use on the rates of specific bacteremias in the total population of HIV-infected patients who are either receiving or not receiving the agent.
Advances in antiretroviral therapy have resulted in immunologic restoration for many HIV-infected patients. With fewer opportunistic infections and a reduced need for prophylactic antimicrobials and therapeutic interventions such as chronic IVCs, it is likely that there will be a reduction in the incidence of bacteremia among those who have responded to such treatment. The ecology and susceptibilities of their blood isolates may become more similar to those of the general community.
Acknowledgments
We thank Stacy Hoshaw-Woodard for assistance with the statistical analysis and Lynn Tolbert for assistance with patient charts.
This work was supported by grant AI25924 (Adult AIDS Clinical Trials Group) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bouza, E., and M. Rodriguez-Creixems. 1999. Bacteremic infections in the HIV-infected patient and recurrent bacteremia. Clin. Microbiol. Infect. 5(Suppl. 2):33-39.
- 2.Buskin, S. E., L. M. Newcomer, L. A. Koutsky, T. M. Hooton, D. H. Spach, and S. G. Hopkins. 1999. Effect of trimethoprim-sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis on bacterial illness, Pneumocystis carinii pneumonia, and death in persons with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:201-206. [DOI] [PubMed] [Google Scholar]
- 3.Dekkar, A. W., M. Rozenberg-Arska, J. J. Sixma, and J. Verhoef. 1981. Prevention of infection by trimethoprim-sulfamethoxazole plus amphotericin B in patients with acute nonlymphocytic leukaemia. Ann. Intern. Med. 95:555-559. [DOI] [PubMed] [Google Scholar]
- 4.Edge, M. D., and D. Rimland. 1996. Community-acquired bacteremia in HIV-positive patients: protective benefit of co-trimoxazole. AIDS 10:1635-1639. [DOI] [PubMed] [Google Scholar]
- 5.Finland, M. 1970. Changing ecology of bacterial infections as related to antibacterial therapy. J. Infect. Dis. 122:419-431. [DOI] [PubMed] [Google Scholar]
- 6.Finland, M. 1972. Changing patterns of susceptibility of common bacterial pathogens to antimicrobial agents. Ann. Intern. Med. 76:1009-1036. [DOI] [PubMed] [Google Scholar]
- 7.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 8.Gurwith, M. J., J. L. Brunton, B. A. Lank, G. K. M. Harding, and A. R. Ronald. 1979. A prospective controlled investigation of prophylactic trimethoprim/sulfamethoxazole in hospitalized granulocytopenic patients. Am. J. Med. 66:248-256. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, W. D., J. Feinberg, D. M. Finkelstein, M. E. Power, W. He, C. Kaczka, P. T. Frame, M. Holmes, H. Waskin, R. J. Fass, W. G. Powderly, R. T. Steigbigel, A. Zuger, and R. S. Holzman. 1992. A controlled trial of trimethoprim-sulfamethoxazole or aerosolized pentamidine for secondary prophylaxis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trials Group Protocol 021. N. Engl. J. Med. 327:1842-1848. [DOI] [PubMed] [Google Scholar]
- 10.Hughes, W. T., S. Kuhn, S. Chaudhary, S. Feldman, M. Verzosa, J. A. Rhomes, C. Pratt, and S. L. George. 1977. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. N. Engl. J. Med. 297:1419-1426. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi, R., P. Costigliola, E. Ricchi, and F. Chiodo. 1993. Sepsis-bacteraemia and other infections due to non-opportunistic bacterial pathogens in a consecutive series of 788 patients hospitalized for HIV infection. Clin. Ter. 143:279-290. [PubMed] [Google Scholar]
- 12.Martin, J. N., D. A. Rose, W. K. Hadley, F. Perdreau-Remington, P. K. Lam, and J. L. Gerberding. 1999. Emergence of trimethoprim-sulfamethoxazole resistance in the AIDS era. J. Infect. Dis. 180:1809-1818. [DOI] [PubMed] [Google Scholar]
- 13.Meyer, C. N., P. Skinhoj, and J. Prag. 1994. Bacteremia in HIV-positive and AIDS patients: incidence, species distribution, risk-factors, outcome, and influence of long-term prophylactic antibiotic treatment. Scand. J. Infect. Dis. 26:635-642. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1993. Approved standard M7-A3. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 15.Northfelt, D. W., and B. Polsky. 1991. Bacteremia in persons with HIV infection. AIDS Clin. Rev. 1991:59-79. [PubMed] [Google Scholar]
- 16.Omeñaca, C., G. Turett, R. Yarrish, M. Astiz, R. Lin, J. W. Kislak, and J. Cadden. 1999. Bacteremia in HIV-infected patients: short-term predictors of mortality. J. Acquir. Immune Defic. Syndr. 22:155-160. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen, G., H. C. Schonheyder, F. H. Steffensen, and H. T. Sorensen. 1999. Risk of resistance related to antibiotic use before admission in patients with community-acquired bacteremia. J. Antimicrob. Chemother. 43:119-126. [DOI] [PubMed] [Google Scholar]
- 18.Tumbarello, M., E. Tacconelli, K. G. Donati, F. Leone, G. Morace, R. Cauda, and L. Ortona. 1998. Nosocomial bloodstream infections in HIV-infected patients: attributable mortality and extension of hospital stay. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:490-497. [DOI] [PubMed] [Google Scholar]