Abstract
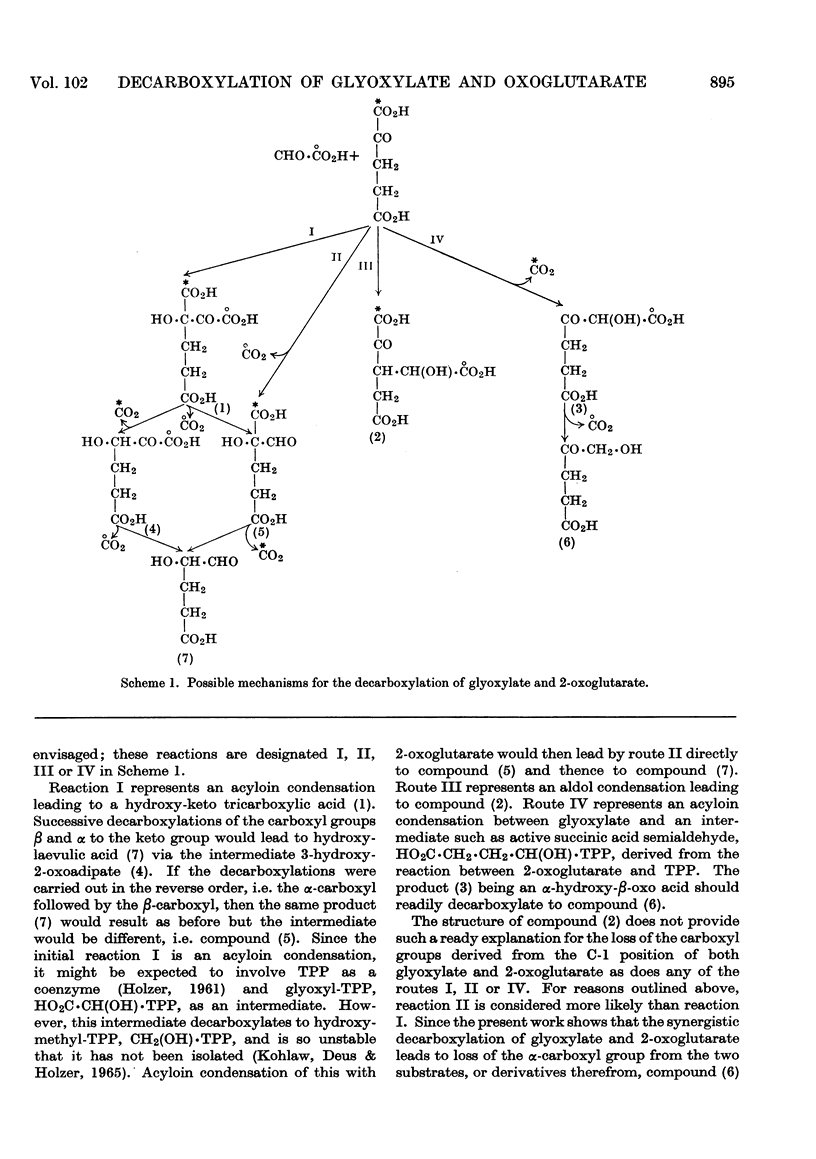
1. An enzyme system that catalyses a synergistic decarboxylation of glyoxylate and 2-oxoglutarate has been purified from pig-liver mitochondria. 2. The purified system is specific for glyoxylate and 2-oxoglutarate as substrates, although in earlier stages of purification glycine and l-glutamate are also active. 3. The reaction is inhibited strongly by EDTA and N-ethylmaleimide. Substrate analogues, present at concentrations equimolar with respect to the substrates, are not effective as inhibitors. 4. The reaction proceeds in the absence of added cofactors. Magnesium chloride, mercaptoethanol and sucrose stimulate the reaction, and stabilize the activity of the enzyme. 5. The pH optimum of the reaction is 7·0. The Km values of glyoxylate and 2-oxoglutarate, at saturating concentration of the corresponding co-substrate, are 16mm and 3·6mm respectively. 6. Isotopic work with specifically labelled [14C]glyoxylate and 2-oxo[14C]-glutarate suggests that the enzyme system catalyses an initial condensation of glyoxylate and 2-oxoglutarate that results in, or leads to, release of C-1 of both substrates as carbon dioxide. C-2 of glyoxylate and C-5 of 2-oxoglutarate do not appear as carbon dioxide. 7. The stoicheiometry of the reaction is complex. During the initial stages of the reaction, more carbon dioxide is recovered from 2-oxoglutarate than from glyoxylate. Subsequently, there is a disproportionate increase with time of carbon dioxide evolution from the carboxyl group of glyoxylate. The excess of decarboxylation of glyoxylate over 2-oxogluturate is further increased by treatment of reaction products with acid.
Full text
PDF

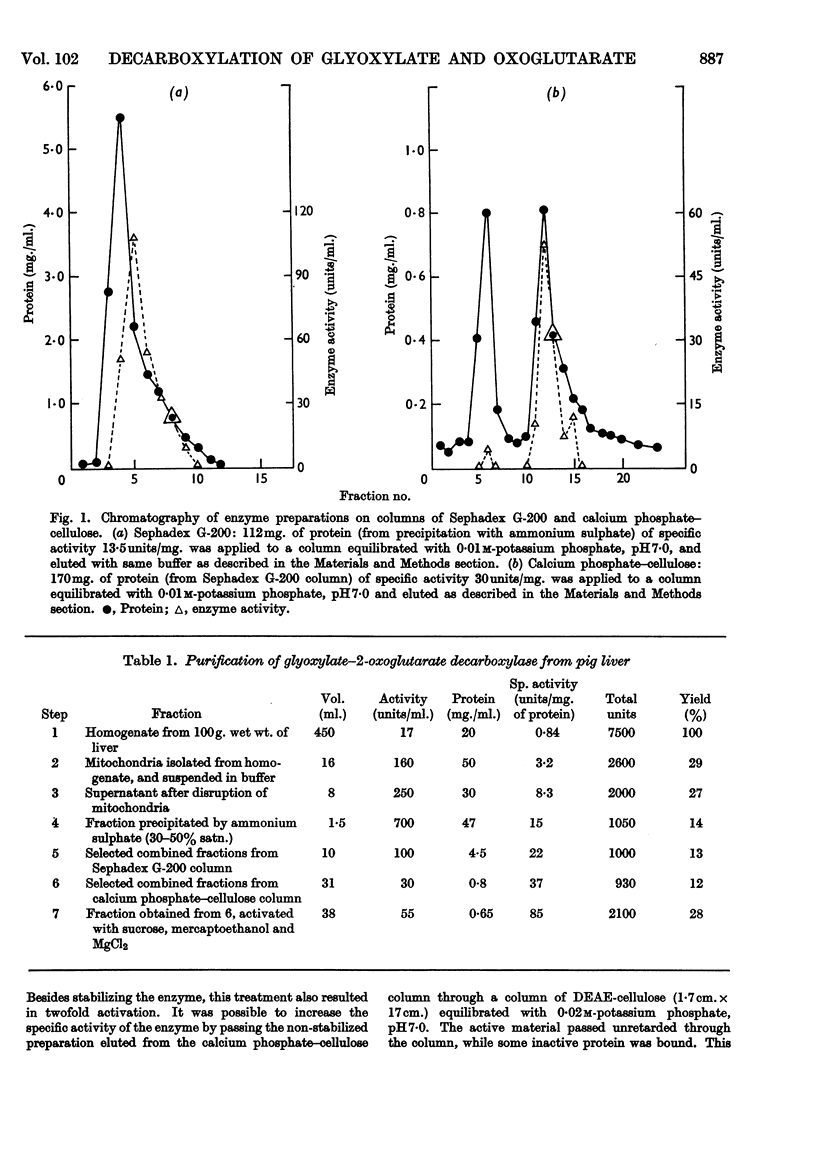

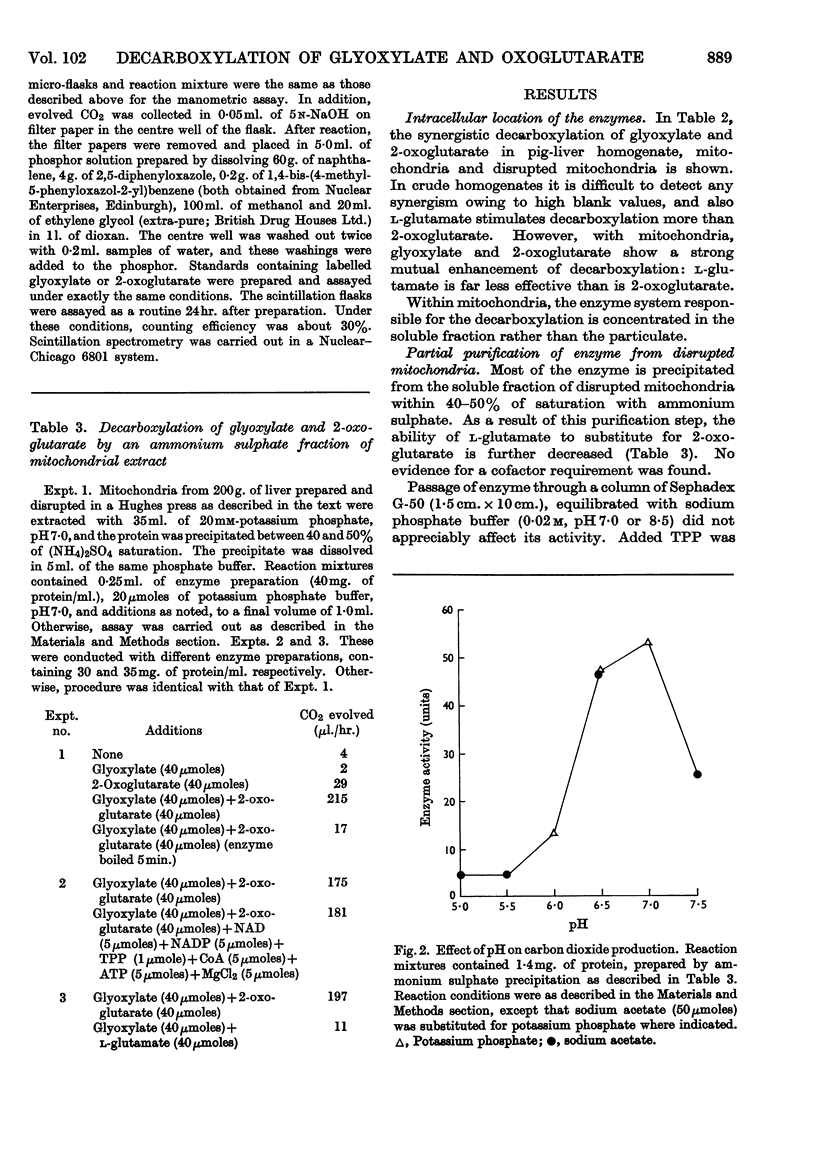

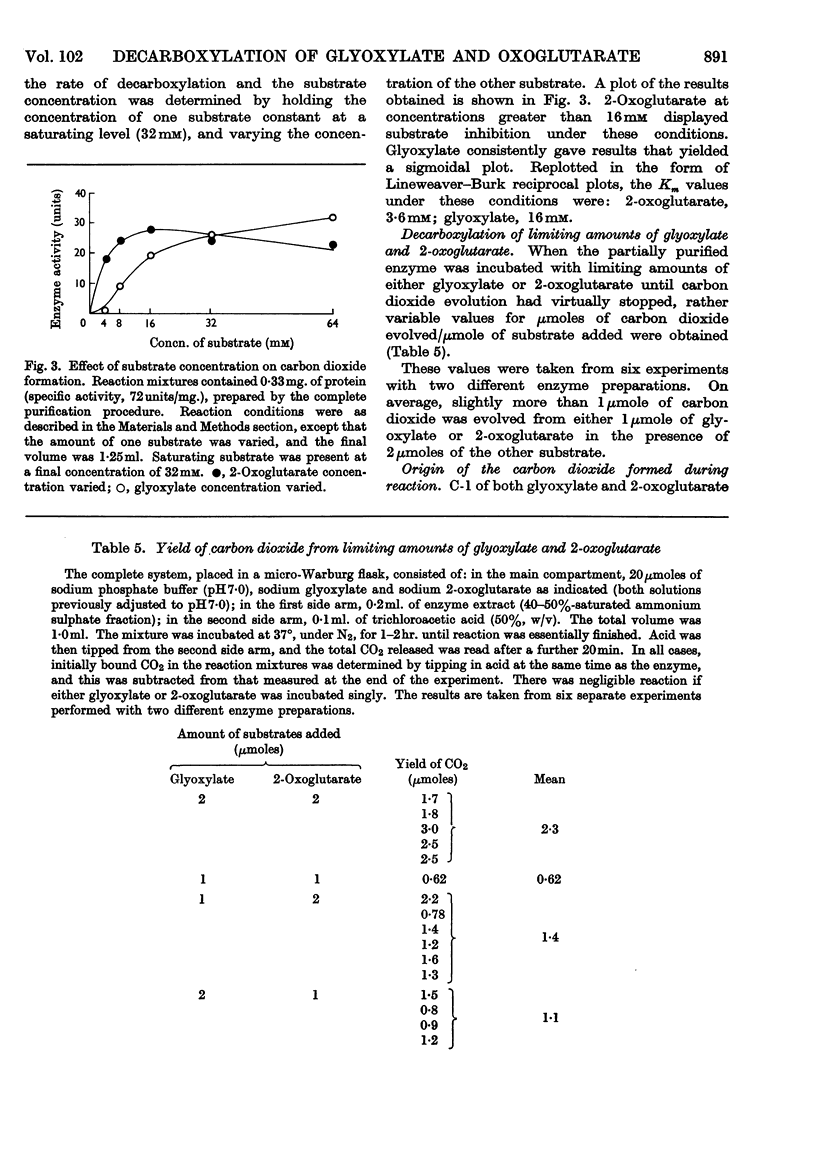

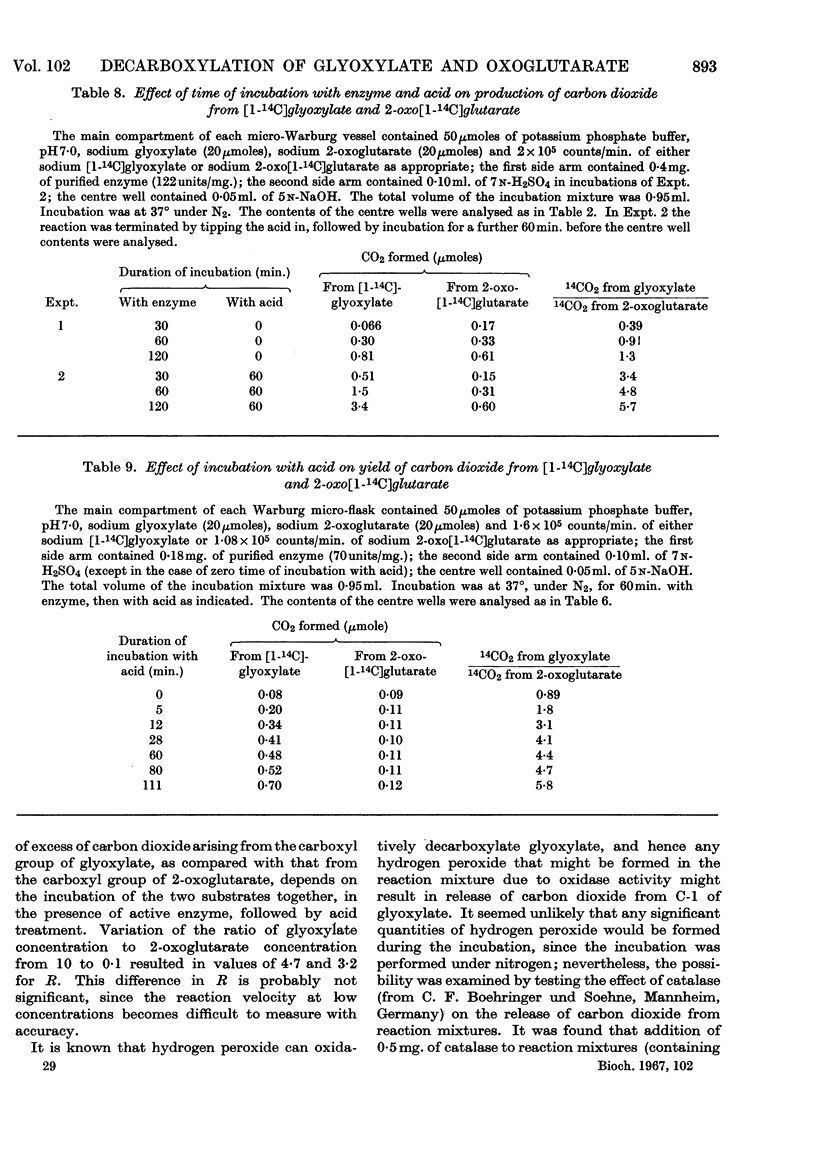


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMMARATA P. S., COHEN P. P. The scope of the transamination reaction in animal tissues. J Biol Chem. 1950 Nov;187(1):439–452. [PubMed] [Google Scholar]
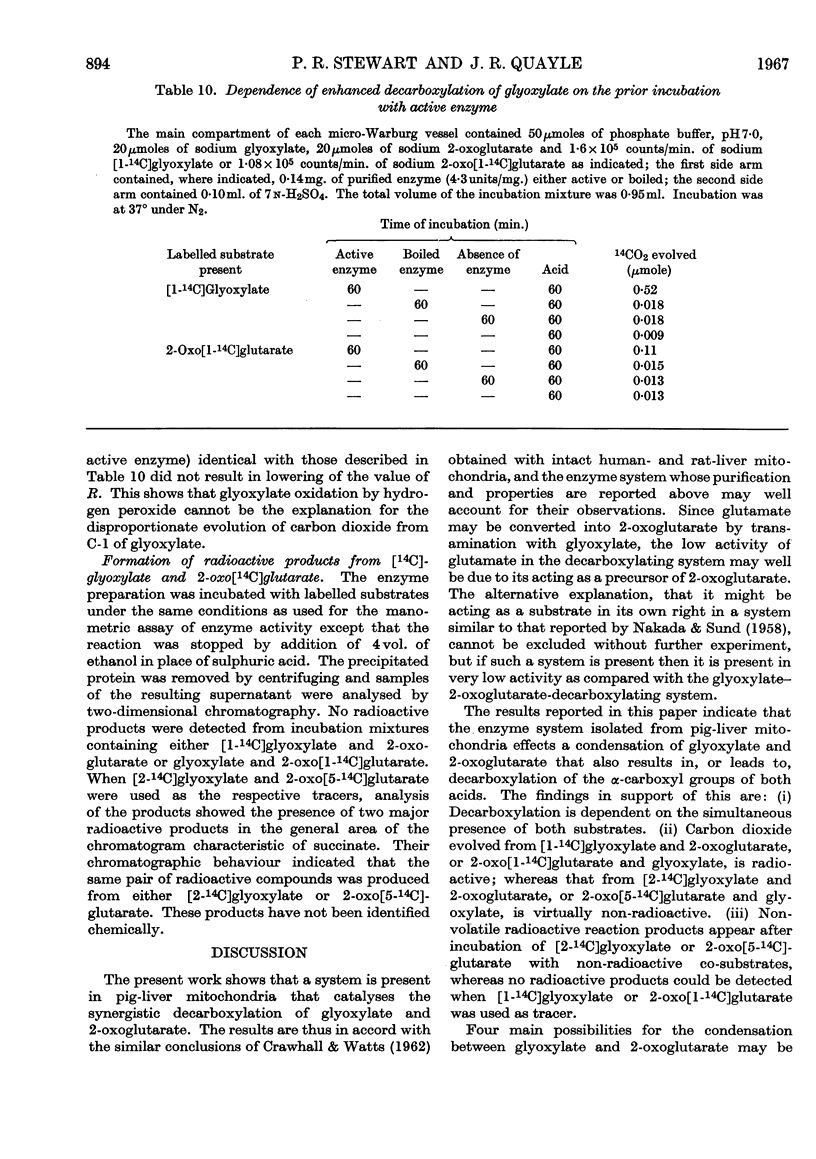
- Crawhall J. C., Watts R. W. The metabolism of glyoxylate by human- and rat-liver mitochondria. Biochem J. 1962 Oct;85(1):163–171. doi: 10.1042/bj0850163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKKER E. E., MAITRA U. Conversion of gamma-hydroxyglutamate to glyoxylate and alanine; purification and properties of the enzyme system. J Biol Chem. 1962 Jul;237:2218–2227. [PubMed] [Google Scholar]
- Ellington E. V., Mellanby J., Williamson D. H. The non-enzymic condensation of acetoacetate and glyoxylate: an explanation for the antiketogenic effect of glycolaldehyde and related compounds. Biochem J. 1964 May;91(2):352–356. doi: 10.1042/bj0910352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING L., CROSBIE G. W. Non-enzymic transamination between glycine and glyoxylate. Biochim Biophys Acta. 1960 Sep 9;43:139–140. doi: 10.1016/0006-3002(60)90421-2. [DOI] [PubMed] [Google Scholar]
- FRANKE W., JILGE G. [On the enzymatic decomposition of C2-acids by microorganisms. II. On an enzyme condensing glyoxylic acid with alpha-ketoglutaric acid from Aspergillus niger]. Arch Mikrobiol. 1961;39:88–94. [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. Substrate competition in the respiration of animal tissues. The metabolic interactions of pyruvate and alpha-oxoglutarate in rat-liver homogenates. Biochem J. 1963 Mar;86:432–446. doi: 10.1042/bj0860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- KOHLHAW G., DEUS B., HOLZER H. ENZYMATIC PREPARATION, STRUCTURE, AND PROPERTIES OF THIAMINE PYROPHOSPHATE-ACTIVATED FORMALDEHYDE. J Biol Chem. 1965 May;240:2135–2141. [PubMed] [Google Scholar]
- KURATOMI K., FUKUNAGA K. A new enzymic reaction concerned in the metabolism of beta-hydroxyglutamic acid. Biochim Biophys Acta. 1960 Oct 7;43:562–563. doi: 10.1016/0006-3002(60)90487-x. [DOI] [PubMed] [Google Scholar]
- KURATOMI K., FUKUNAGA K. THE METABOLISM OF GAMMA-HYDROXYGLUTAMATE IN RAT LIVER. I. ENZYMIC SYNTHESIS OF GAMMA-HYDROXY-ALPHA-KETOGLUTARATE FROM PYRUVATE AND GLYOXYLATE. Biochim Biophys Acta. 1963 Dec 13;78:617–628. doi: 10.1016/0006-3002(63)91027-8. [DOI] [PubMed] [Google Scholar]
- Koch J., Stokstad E. L. Partial purification of a 2-oxo-glutarate:glyoxylate carboligase from rat liver mitochondria. Biochem Biophys Res Commun. 1966 Jun 13;23(5):585–591. doi: 10.1016/0006-291x(66)90439-6. [DOI] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA H. I., FRIEDMANN B., WEINHOUSE S. Pathways of glycine catabolism in rat liver. J Biol Chem. 1955 Oct;216(2):583–592. [PubMed] [Google Scholar]
- NAKADA H. I. GLUTAMIC-GLYCINE TRANSAMINASE FROM RAT LIVER. J Biol Chem. 1964 Feb;239:468–471. [PubMed] [Google Scholar]
- NAKADA H. I., SUND L. P. Glyoxylic acid oxidation by rat liver. J Biol Chem. 1958 Jul;233(1):8–13. [PubMed] [Google Scholar]
- NAKADA H. I., WEINHOUSE S. Non-enzymatic transamination with glyoxylic acid and various amino acids. J Biol Chem. 1953 Oct;204(2):831–836. [PubMed] [Google Scholar]
- NAKADA H. I., WEINHOUSE S. Studies of glycine oxidation in rat tissues. Arch Biochem Biophys. 1953 Feb;42(2):257–270. doi: 10.1016/0003-9861(53)90356-7. [DOI] [PubMed] [Google Scholar]
- RUFFO A., ADINOLFI A., BUDILLON G., CAPOBIANCO G. Control of the citric acid cycle by glyoxylate. 2. Mechanism of the inhibition of respiration in liver and kidney particles. Biochem J. 1962 Dec;85:593–600. doi: 10.1042/bj0850593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWINGLE S. M., TISELIUS A. Tricalcium phosphate as an adsorbent in the chromatography of proteins. Biochem J. 1951 Feb;48(2):171–174. doi: 10.1042/bj0480171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINHOUSE S., FRIEDMANN B. Metabolism of labeled 2-carbon acids in the intact rat. J Biol Chem. 1951 Aug;191(2):707–717. [PubMed] [Google Scholar]


