Abstract
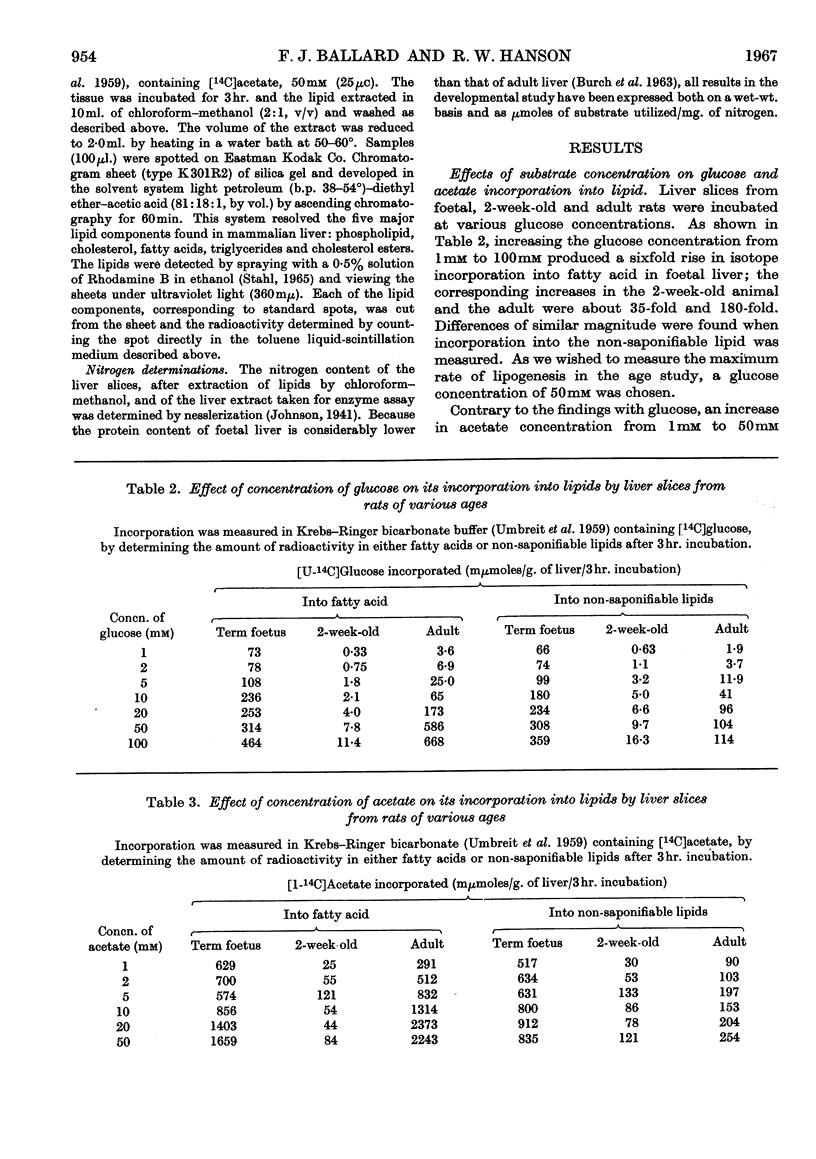
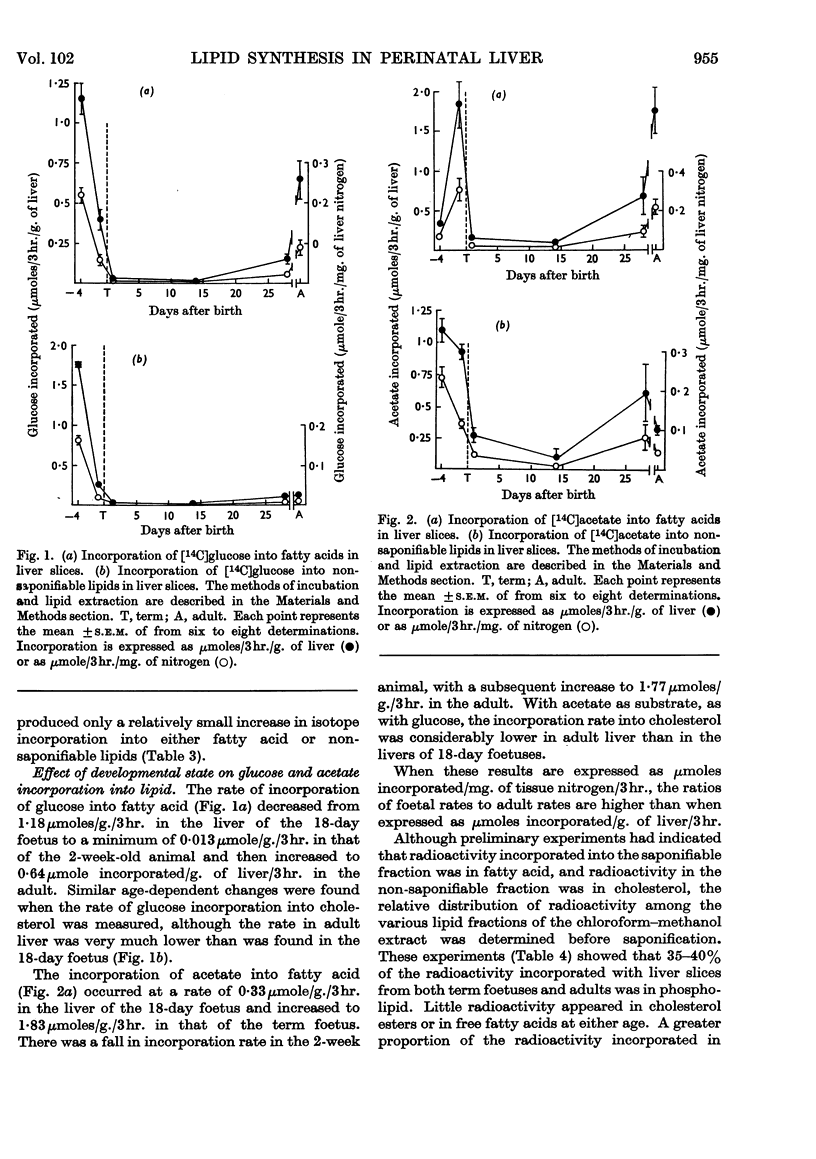
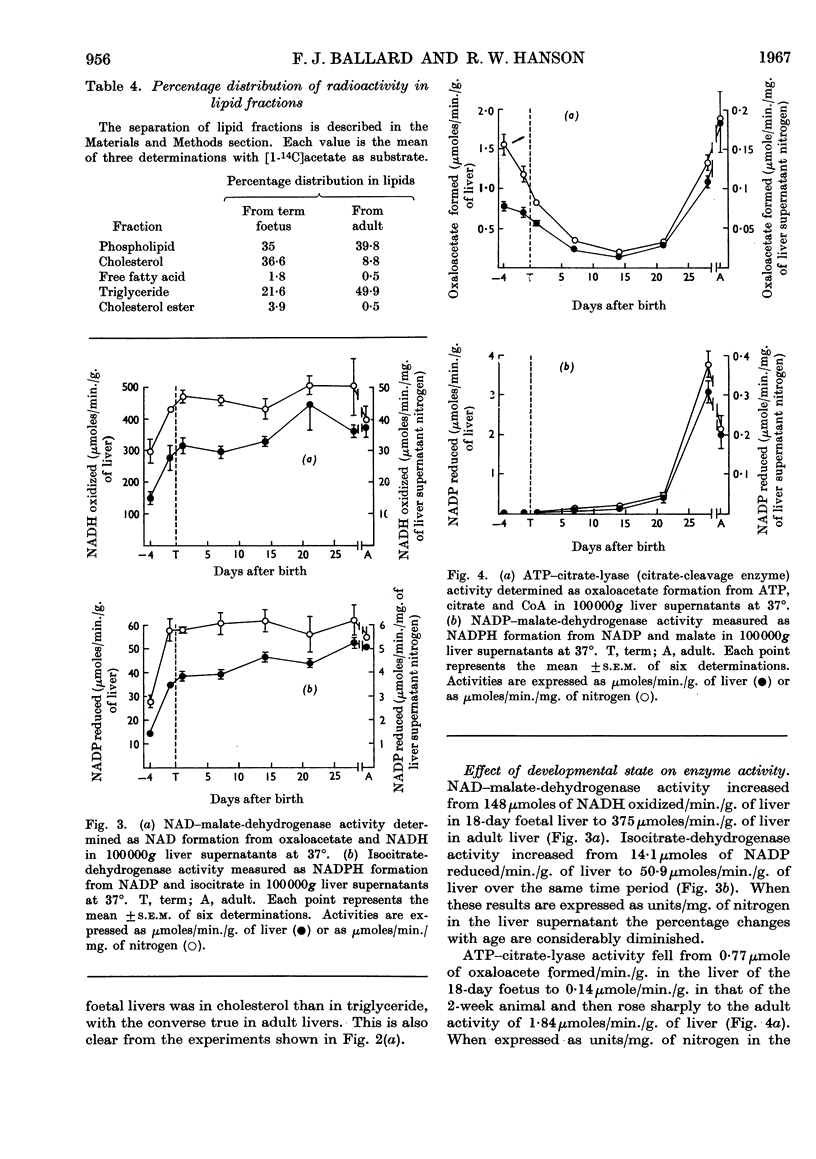
1. Lipogenesis, as measured by the incorporation of 14C-labelled glucose or acetate into fatty acids in liver slices, is high in foetal and adult rat liver but is low in the liver of the suckling rat, especially with glucose as substrate. 2. The rate of synthesis of non-saponifiable lipids from glucose is about 15 times as great in the liver of the 18-day foetus as in adult liver. Activity in the newborn is negligible. 3. Glucose incorporation into fat is strongly concentration-dependent in liver slices from the adult and 2-week-old rat, but less markedly so in liver slices from the foetus. 4. Changes in the activity of hepatic citrate-cleavage enzyme (ATP–citrate lyase) occur in parallel with the changes in the extent of fatty acid formation, supporting the participation of this enzyme in lipogenesis. However, NADP–malate dehydrogenase, a potential source of reduced nucleotide coenzyme for lipogenesis in the adult, could not be detected in foetal rat liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLARD F. J., OLIVER I. T. CARBOHYDRATE METABOLISM IN LIVER FROM FOETAL AND NEONATAL SHEEP. Biochem J. 1965 Apr;95:191–200. doi: 10.1042/bj0950191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLARD F. J., OLIVER I. T. Glycogen metabolism in embryonic chick and neonatal rat liver. Biochim Biophys Acta. 1963 Jun 4;71:578–588. doi: 10.1016/0006-3002(63)91130-2. [DOI] [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., KUHLMAN A. M., SKERJANCE J., DIAMANT E. J., LOWRY S. R., VON DIPPE P. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J Biol Chem. 1963 Jul;238:2267–2273. [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. Ketohexokinase, isoenzymes of glucokinase and glycogen synthesis from hexoses in neonatal rat liver. Biochem J. 1964 Feb;90(2):261–268. doi: 10.1042/bj0900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. The effect of concentration on glucose phosphorylation and incorporation into glycogen in the livers of foetal and adult rats and sheep. Biochem J. 1964 Jul;92(1):131–136. doi: 10.1042/bj0920131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, HASTINGS A. B., ASHMORE J., ZOTTU S. Studies on carbohydrate metabolism in rat liver slices. X. Factors in the regulation of pathways of glucose metabolism. J Biol Chem. 1958 Jan;230(1):125–135. [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- Dawkins M. J. Biochemical aspects of developing function in newborn mammalian liver. Br Med Bull. 1966 Jan;22(1):27–33. doi: 10.1093/oxfordjournals.bmb.a070432. [DOI] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREEDMAN A. D., NEMETH A. M. The metabolism of pyruvate in the tricarboxylic acid cycle of developing mammalian liver. J Biol Chem. 1961 Dec;236:3083–3085. [PubMed] [Google Scholar]
- HANSON R. W. INTERRELATIONSHIP OF KETONE BODY METABOLISM AND GLUCOSE UTILIZATION BY ADIPOSE TISSUE IN VITRO. Arch Biochem Biophys. 1965 Jan;109:98–103. doi: 10.1016/0003-9861(65)90292-4. [DOI] [PubMed] [Google Scholar]
- HASTINGS A. B., TENG C. T., NESBETT F. B., SINEX F. M. Studies on carbohydrate metabolism in rat liver slices. I. The effect of cations in the media. J Biol Chem. 1952 Jan;194(1):69–81. [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS N., KING H. K., BROWN S. J. Substrate attachment in enzymes. The interaction of pyridoxal phosphate with amino acids. Biochem J. 1962 Jul;84:118–124. doi: 10.1042/bj0840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea M. A., Walker D. G. The metabolism of glucose 6-phosphate in developing mammalian tissues. Biochem J. 1964 Jun;91(3):417–424. doi: 10.1042/bj0910417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER A. F., LOWENSTEIN J. M. The supply of precursors for the synthesis of fatty acids. J Biol Chem. 1962 Dec;237:3640–3648. [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. PATTERNS OF DIETARY AND HORMONAL INDUCTION OF CERTAIN NADP-LINKED LIVER ENZYMES. Am J Physiol. 1964 Feb;206:357–361. doi: 10.1152/ajplegacy.1964.206.2.357. [DOI] [PubMed] [Google Scholar]
- VILLEE C. A., HAGERMAN D. D. Effect of oxygen deprivation on the metabolism of fetal and adult tissues. Am J Physiol. 1958 Sep;194(3):457–464. doi: 10.1152/ajplegacy.1958.194.3.457. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


