Abstract
Persistent infections with Chlamydia pneumoniae have been implicated in the development of chronic diseases, such as atherosclerosis and asthma. Although azithromycin, clarithromycin, and levofloxacin are frequently used for the treatment of respiratory C. pneumoniae infections, little is known about the dose and duration of therapy needed to treat a putative chronic C. pneumoniae infection. In this study, we investigated the effect of prolonged treatment with azithromycin, clarithromycin, or levofloxacin on the viability of C. pneumoniae and cytokine production in an in vitro model of continuous infection. We found that a 30-day treatment with azithromycin, clarithromycin, and levofloxacin at concentrations comparable to those achieved in the pulmonary epithelial lining fluid reduced but did not eliminate C. pneumoniae in continuously infected HEp-2 cells. All three antibiotics decreased levels of interleukin-6 (IL-6) and IL-8 in HEp-2 cells, but this effect appeared to be secondary to the antichlamydial activity, as the cytokine levels correlated with the concentrations of microorganisms. The levels of IL-1β, IL-4, IL-10, tumor necrosis factor alpha, and gamma interferon were too low to assess the effect of antibiotics. These data suggest that the dosage and duration of antibiotic therapy currently being used may not be sufficient to eradicate a putative chronic C. pneumoniae infection.
Chlamydia pneumoniae is capable of causing chronic, persistent, and asymptomatic infections. Persistent C. pneumoniae infection has been implicated in the development of chronic diseases in humans, including atherosclerosis and asthma (33). Azithromycin, clarithromycin, and levofloxacin are frequently used for the treatment of C. pneumoniae respiratory infections. Several antibiotic treatment trials for the prevention of secondary cardiovascular events in patients with coronary artery disease, using prolonged courses of treatment, have been published or are underway (1, 11, 12). However, little is known about the dose and duration of therapy needed to treat a putative chronic C. pneumoniae vascular infection. Microbiologic failure has been described in C. pneumoniae infections, even after prolonged courses of azithromycin, erythromycin, and doxycycline (4, 14).
We previously reported that treatment with azithromycin and ofloxacin, at concentrations up to four times the MIC, reduced but did not completely eliminate the organism after 6 days of treatment in an in vitro model of continuous C. pneumoniae infection (23). In the present study, we investigated the effect of higher concentrations and a longer duration of treatment with azithromycin, clarithromycin, or levofloxacin on the growth of C. pneumoniae in this model. In addition, we investigated the effect of these drugs on production of inflammatory cytokines in this model.
MATERIALS AND METHODS
Continuous C. pneumoniae infection in vitro.
Confluent HEp-2 cell monolayers inoculated with C. pneumoniae TW-183 (ATCC VR-2282) and CM-1 (ATCC VR-1360) were maintained for over 4 years without centrifugation or addition of cycloheximide or fresh cells, as previously described (23).
Antibiotic activity assay.
Continuously infected HEp-2 cells were seeded into 12-well plates the day prior to the experiment and incubated at 35°C. On day 0, the supernatant media of all infected cells were replaced with media containing one of the following antimicrobials: 4 μg of azithromycin/ml, 16 μg of levofloxacin/ml, or 64 μg of clarithromycin/ml. These concentrations are comparable to those achieved in the pulmonary epithelial lining fluid of patients and volunteers (2, 29). Every third day during the experiment, the media were replaced with fresh media containing the same antimicrobial at the above concentrations. Infected cells and supernatants were collected at 0, 6, 12, 18, 24, and 30 days and frozen. The inclusion-forming units (IFU) per milliliter for every time point were determined as previously described (23). Briefly, infected cells were defrosted and ultrasonicated. Ten-fold dilutions from each well were inoculated onto fresh HEp-2 cells in 96-well plates. The plates were centrifuged at 1,700 × g, incubated for 72 h, fixed, and stained with fluorescein isothiocyanate-conjugated genus-specific murine monoclonal antibodies. The IFU per milliliter for every time point, isolate, and drug was calculated. The reduction of IFU was calculated compared to controls with no antibiotics added.
Statistical analysis.
Two-way analysis of variance (ANOVA) was conducted separately for each isolate, with antimicrobials and time points as factors. Post-hoc testing was then performed for each isolate, using Dunnetts test to compare antimicrobial effects with the no-drug controls at each time-point. The significance level was set at 0.05.
Cytokine production assay.
Cytokines interleukin-1β (IL-1β), IL-4, IL-6, IL-8, IL-10, tumor necrosis factor alpha (TNF-α), and gamma interferon were assayed by sandwich enzyme-linked immunosorbent assay (ELISA) (Cytoscreen; Biosource International, Camarillo, Calif.) according to the manufacturer's instructions. Uninfected HEp-2 cells were used as controls.
RESULTS
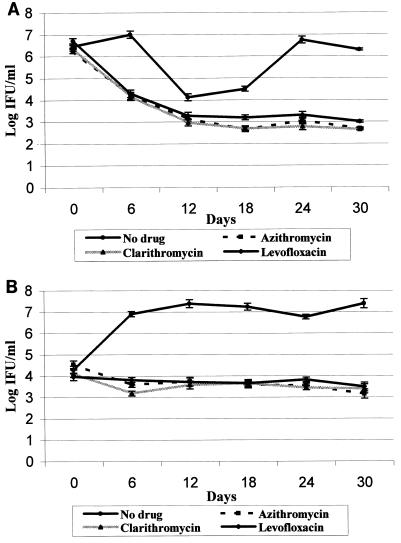
The results of the treatment of continuous C. pneumoniae cultures with azithromycin, clarithromycin, and levofloxacin are shown in Fig. 1.During the first 12 days of treatment, the titers of CM-1 decreased from 106 to 107 IFU/ml to 102 to 103 IFU/ml with all three antibiotics (Fig. 1A). From days 12 to 30, the titers of CM-1 cells remained stable at 102 to 103 IFU/ml. Titers of TW-183 decreased within 6 days, from 104 to 103 IFU/ml, and remained at that level until the end of the experiment (Fig. 1B). The HEp-2 cells in CM-1 control cultures (where the initial chlamydia concentration was higher than that of TW-183) underwent a cycle of cell lysis and regrowth during the experiment that consequently led to the fall in CM-1 titers on days 12 and 18, as seen in Fig. 1A. These infection cycles, with intervals of 7 to 21 days, are characteristic of continuous C. pneumoniae infection in vitro (22). Statistical analysis demonstrated strong antimicrobial-by-time interactions (P < 0.0001) for both isolates. There were no significant differences between C. pneumoniae concentrations in cultures treated with antimicrobials and controls on day 0, whereas for every subsequent time point, each titer in cultures with antimicrobials differed significantly from titers in controls.
FIG. 1.
Effect of azithromycin (4 μg/ml), clarithromycin (64 μg/ml), and levofloxacin (16 μg/ml) on continuous CM-1 (A) and TW-183 (B) infection.
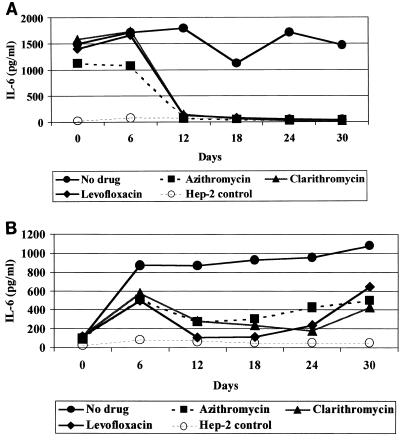
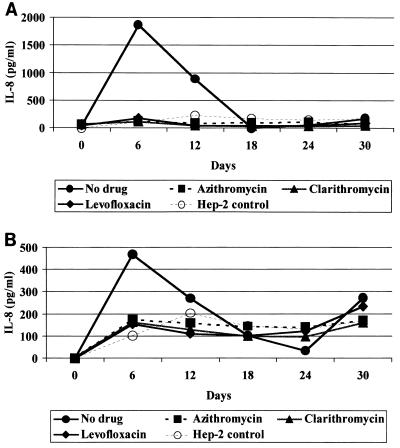
Both isolates of C. pneumoniae stimulated significant production of IL-6 and IL-8. Levels of IL-6 were 13 to 21 times higher than those in uninfected controls and were elevated throughout the experiment (Fig. 2).IL-8 reached peak levels on day 6 and decreased to the levels of uninfected controls by day 18 (Fig. 3).Treatment with all three antibiotics decreased levels of IL-6 and IL-8 by days 12 and 18, respectively, to the levels detected in uninfected HEp-2 cells with the exception of IL-6 levels in continuous TW-183 cultures that still were approximately 1.6 to 13.4 times higher than those of controls (Fig. 2B). The other cytokines were either undetectable or present at levels too low to assess the effect of antibiotics.
FIG. 2.
Effect of azithromycin (4 μg/ml), clarithromycin (64 μg/ml), and levofloxacin (16 μg/ml) on levels of IL-6 in supernatants of CM-1 (A) and TW-183 (B) infected cells.
FIG. 3.
Effect of azithromycin (4 μg/ml), clarithromycin (64 μg/ml), and levofloxacin (16 μg/ml) on levels of IL-8 in the supernatants of CM-1 (A) and TW-183 (B) infected cells.
DISCUSSION
Methods currently used for culturing C. pneumoniae and in in vitro susceptibility studies are not analogous to the infection as it occurs in vivo. We established an in vitro model of continuous C. pneumoniae infection with HEp-2 cells which had remained persistently infected for over 4 years without addition of fresh chlamydia or host cells, addition of cycloheximide, or centrifugation (23). Ultrastructural studies of the continuously infected cells revealed the presence of a subpopulation of abnormal inclusions, which were very similar in appearance to persistent forms induced after treatment with gamma interferon (22). Therefore, this model may more accurately reflect interactions between chlamydia and host cells and, hence, be a better model for in vitro susceptibility studies of C. pneumoniae.
The results of this study demonstrated that prolonged treatment with azithromycin, clarithromycin, and levofloxacin at concentrations achieved in the epithelial lining fluid reduced but did not eliminate C. pneumoniae from continuously infected host cells.
Galasso and Manire (7) were the first researchers to employ a continuous-infection model for antibiotic activity testing. They utilized HeLa cells continuously infected with Chlamydia psittaci to determine the effect of penicillin, tetracycline, and chloramphenicol. They found that 500 U of penicillin/ml suppressed the chlamydial growth, but even prolonged treatment for 100 days failed to eliminate the organism. More than 14 days of treatment with 10 μg of tetracycline/ml or 21 days with 25 and 100 μg of chloramphenicol/ml was necessary to suppress chlamydial growth to undetectable levels. Dreses-Werringloer et al. (6) recently reported similar observations on the effect of ciprofloxacin and ofloxacin on established (2 to 3 days postinoculation) C. trachomatis infection. They found that both drugs, at concentrations which exceeded the minimal bactericidal concentration (0.5 μg of ciprofloxacin/ml, 1.0 and 2.0 μg of ofloxacin/ml), failed to eradicate C. trachomatis from infected HEp-2 cells and also induced persistent infection characterized by a low number of small aberrant inclusions present through 20 days of culture. After the removal of ciprofloxacin from the media 10 or 14 days postinfection, the persistent chlamydia reverted to overt growth.
The results of this study bring up some important issues regarding the use of antibiotics, including azithromycin, for secondary prevention of cardiac morbidity (1, 10, 13). The dosages of azithromycin being used are 500 or 600 mg/day for 3 and 6 days followed by weekly doses of 500 to 600 mg for periods of 3 months to 1 year. Based on the data presented here, it would appear unlikely that these dosage regimens would eliminate C. pneumoniae from an intravascular focus. The standard respiratory dosage of 1.5 g of azithromycin over 5 days had only 70 and 83% efficacy in eradicating C. pneumoniae from the nasopharynx of culture-positive adults and children, respectively, with community-acquired pneumonia (28). Data are similar for other antibiotics. Block et al. (3) found that a 10-day treatment with erythromycin or clarithromycin suspension eradicated C. pneumoniae from the nasopharynx of 86 and 79% of culture-positive children with community-acquired pneumonia, respectively, despite the fact that clarithromycin was four times more active in vitro (15). The results of two pneumonia treatment studies in adults, which evaluated levofloxacin and moxifloxacin, found eradication rates of 70 to 80% (16, 17). Dessus-Babus et al. recently described the induction of resistance to ofloxacin and sparfloxacin in Chlamydia trachomatis after serial passing of the organism in subinhibitory concentrations of these drugs (5). Antibiotic resistance has not as yet been described for C. pneumoniae. However, the MICs of three isolates of C. pneumoniae, obtained from two patients with community-acquired pneumonia treated with azithromycin, increased fourfold after treatment, although they were still within the range considered susceptible to the drug (28). It is not clear if it was an isolated event or suggestive of a possible development of persistence. Furthermore, once weekly dosing with azithromycin may result in prolonged exposure to subinhibitory drug levels, leading to the development of resistance in other respiratory bacteria, especially Streptococcus pneumoniae (24, 26).
The existence of persistence also raises a separate important issue for the treatment of C. pneumoniae-associated diseases. Persistent forms generally do not replicate or have reduced activity and therefore may not be susceptible to antibiotics. It is quite possible that the 20 to 30% rate of microbiologic failures in reported C. pneumoniae treatment studies (3, 16, 17, 18) and the ability of C. pneumoniae to survive antibiotic treatment in our experiments may be directly related to the persistent state.
C. pneumoniae can stimulate the production of cytokines, chemokines, and adhesion molecules in various endothelial and epithelial cell lines (8, 9, 20, 25, 27). These immunologically active molecules are able to induce and sustain inflammatory process that may play an essential role in the pathogenesis of atherosclerosis (30). Preliminary data demonstrated higher production of some cytokines in the continuous-infection model compared to primary cultures (27). In this study, C. pneumoniae stimulated significant production of IL-6 and IL-8 in the continuously infected HEp-2 cells. These cytokines have been detected in fibrous plaques suggestive of their involvement in the development of atherosclerosis (31).
Macrolides and tetracyclines have been shown to possess anti-inflammatory properties independent of their antimicrobial activity (19, 21, 32). Azithromycin and clarithromycin at concentrations of 1, 5, and 10 μg/ml have been demonstrated to affect in various degree production of IL-1α, IL-1β, IL-6, IL-10, granulocyte-macrophage colony-stimulating factor, and TNF-α by human monocytes (21). Most remarkably, azithromycin resulted in a significant decrease of IL-1a and TNF-α in 100% of individuals and treatment with clarithromycin resulted in a significant decrease of IL-6 and TNF-α in 60 and 86% of individuals, respectively. Similarly, reduction in 6-keto-prostaglandin F1α, NO2, TNF-α, IL-1β, and IL-6 levels has been observed in murine macrophages treated with 5 to 80 μM of azithromycin, clarithromycin, roxithromycin, and erythromycin (19). Although in this study treatment with all three antibiotics decreased levels of IL-6 and IL-8 in the continuous cultures, this effect appeared to be primarily secondary to antichlamydial activity, as the levels of cytokines correlated with the titers of C. pneumoniae.
REFERENCES
- 1.Anderson, J. L., J. B. Muhlestein, J. Carlquis, A. Allen, S. Trehan, C. Nielson, S. Hal, J. Brady, M. Egger, B. Horne, and T. Lim. 1999. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection: the Azithromycin in coronary artery disease: elimination of myocardial infarction with chlamydia (ACADEMIC) study. Circulation 99:1540-1547. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, J. M., D. Honeybourne, D., G. Jevons, N. P. Brenwald, B. Cunningham, and R. J. Wise. 1997. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fiber optic bronchoscopy. J. Antimicrob. Chemother. 40:573-577. [DOI] [PubMed] [Google Scholar]
- 3.Block, S., J. Hedrick, M. R. Hammerschlag, G. H. Cassell, and C. Craft. 1995. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr. Infect. Dis. J. 14:471-477. [DOI] [PubMed] [Google Scholar]
- 4.Dean, D., P. M. Roblin, L. Mandel, J. Schachter, and M. Hammerschlag. 1998. Molecular evaluation of serial isolates from patients with persistent Chlamydia pneumoniae infections, p. 219-223. In R. S. Stephens, G. I. Byrne, G. Christiansen, I. N. Clark, J. T. Grayston, R. G. Rank, G. L. Ridgeway, P. Saikku, J. Schachter, and W. E. Stamm (ed.), Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. USCF, San Francisco, Calif.
- 5.Dessus-Babus, S., C. M. Bebear, A. Charron, C. Bebear, and B. de Barbeyrac. 1998. Sequencing of gyrase and topoisomerase IV quinolone-resistance-determining regions of Chlamydia trachomatis and characterization of quinolone-resistant mutants obtained in vitro. Antimicrob. Agents Chemother. 42:2447-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreses-Werringloer, U., I. Padubrin, B. Jurgens-Saathoff, A. P. Hudson, H. Zeidler, and L. Kohler. 2000. Persistence of Chlamydia trachomatis is induced by ciprofloxacin and ofloxacin in vitro. Antimicrob. Agents Chemother. 44:3288-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galasso, G. J., and G. P. Manire. 1961. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J. Immunol. 86:382-385. [PubMed] [Google Scholar]
- 8.Gaydos, C. A. 2000. Growth in vascular cells and cytokine production by Chlamydia pneumoniae. J. Infect. Dis. 181:S473-S478. [DOI] [PubMed] [Google Scholar]
- 9.Gaydos, C. A., J. T. Summersgill, N. N. Sahney, J. A. Ramirez, and T. C. Quinn. 1996. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic arthery smooth muscle cells. Infect. Immun. 64:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayston, J. T. 1998. Antibiotic treatment of Chlamydia pneumoniae for secondary prevention of cardiovascular events. Circulation 97:1669-1670. [DOI] [PubMed] [Google Scholar]
- 11.Grayston, J. T., L. A. Jackson, W. J. Kennedy, and R. A. Kronmal. 1999. Secondary prevention trials for coronary arthery diseases with antibiotic treatment for Chlamydia pneumoniae: design issues. Am. Heart J. 138:S545-S549. [DOI] [PubMed] [Google Scholar]
- 12.Gupta, S. 1999. Chlamydia pneumoniae, monocyte activation, and azithromycin in coronary heart diseases. Am. Heart J. 138:S539-S541. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, S., E. W. Leatham, D. Carrington, M. A. Mendall, J. C. Kaski, and A. J. Camm. 1997. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 96:404-407. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschlag, M. R., K. Chirgwin, P. M. Roblin, M. Gelling, W. Dumornay, L. Mandel, P. Smith, and J. Schachter. 1992. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin. Infect. Dis. 14:178-182. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschlag, M. R., K. K. Qumei, and P. M. Roblin. 1992. In vitro activities of azithromycin, clarithromycin, l-ofloxacin, and other antibiotics Chlamydia pneumoniae. Antimicrob. Agents Chemother. 36:1573-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschlag, M. R., and P. M. Roblin. 2000. Microbiological efficacy of levofloxacin for treatment of community-acquired pneumonia due to Chlamydia pneumoniae. Antimicrob. Agents Chemother. 44:1409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschlag, M. R., and P. M. Roblin. 2000. Microbiologic efficacy of moxifloxacin for the treatment of community-acquired pneumonia due to Chlamydia pneumoniae. Int. J. Antimicrob. Agents 15:149-152. [DOI] [PubMed] [Google Scholar]
- 18.Harris J-A, A. Kolokathis, M. Campbell, G. H. Cassell, and M. R. Hammerschlag. 1998. Safety and efficacy of azithromycin in the treatment of community acquired pneumonia in children. Pediatr. Infect. Dis. J. 17:865-871. [DOI] [PubMed] [Google Scholar]
- 19.Ianaro, A., A. Ialenti, P. Maffia, L. Sautebin, L. Rombola, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. J. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. Pharmacol. Exp. Ther. 292:156-163. [PubMed] [Google Scholar]
- 20.Kaukoranta-Tolvanen, S. S., A. M. Teppo, K. Laitinen, P. Saikku, K. Linnavuori, and M. Leinonnen. 1996. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb. Pathog. 21:215-221. [DOI] [PubMed] [Google Scholar]
- 21.Khan, A. A., T. R. Slifer, F. G. Araujo, and J. S. Remington. 1999. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int. J. Antimicrob. Agents 11:121-132. [DOI] [PubMed] [Google Scholar]
- 22.Kutlin, A., C. Flegg, D. Stenzel, T. Reznik, P. M. Roblin, S. Mathews, P. Timms, and M. R. Hammerschlag. 2001. Ultrastructural study of Chlamydia pneumoniae in a continuous infection model. J. Clin. Microbiol. 39:3721-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutlin, A., P. M. Roblin, and M. R. Hammerschlag. 1999. In vitro activity of azithromycin and ofloxacin against Chlamydia pneumoniae in a continuous infection model. Antimicrob. Agents Chemother. 43:2268-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leach, A. J., T. M. Shelby-James, M. Mayo, M. Gratten, A. C. Laming, B. J. Currie, and J. D. Mathews. 1997. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin. Infect. Dis. 24:356-362. [DOI] [PubMed] [Google Scholar]
- 25.Netea, M. G., C. H. Selzman, B. J. Kullberg, J. M. D. Galama, A. Weinberg, A. F. H. Stalenhoef, J. W. M. Van der Meer, and C. A. Dinarello. 2000. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur. J. Immunol. 30:541-549. [DOI] [PubMed] [Google Scholar]
- 26.Pankuch, G. A., S. A. Jueneman, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 1998. In vitro selection of resistance to four beta-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2914-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roblin, P. M., A. Kutlin, and M. R. Hammerschlag. 1996. Production of IL-4 and IL-6 in HEp-2 cells infected with Chlamydia pneumoniae. Infect. Dis. Obstet. Gynecol. 4:195-196. [Google Scholar]
- 28.Roblin, P. M., and M. R. Hammerschlag. 1998. Microbiologic efficacy of azithromycin and susceptibility to azithromycin of isolates of Chlamydia pneumoniae from adults and children with community acquired pneumonia. Antimicrob. Agents Chemother. 42:194-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross, R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990's. Nature 362:801-808. [DOI] [PubMed] [Google Scholar]
- 31.Rus, H. G., R. Vlaicu, and F. Niculescu. 1996. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127:263-271. [DOI] [PubMed] [Google Scholar]
- 32.Scaglione, F., and G. Rossoni. 1998. Comparative ant-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J. Antimicrob. Chemother. 41(Suppl. B):47-50. [DOI] [PubMed] [Google Scholar]
- 33.Wong, Y-K., P. G. Gallagher, and M. E. Ward. 1999. Chlamydia pneumoniae and atherosclerosis. Heart 81:232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]