Abstract
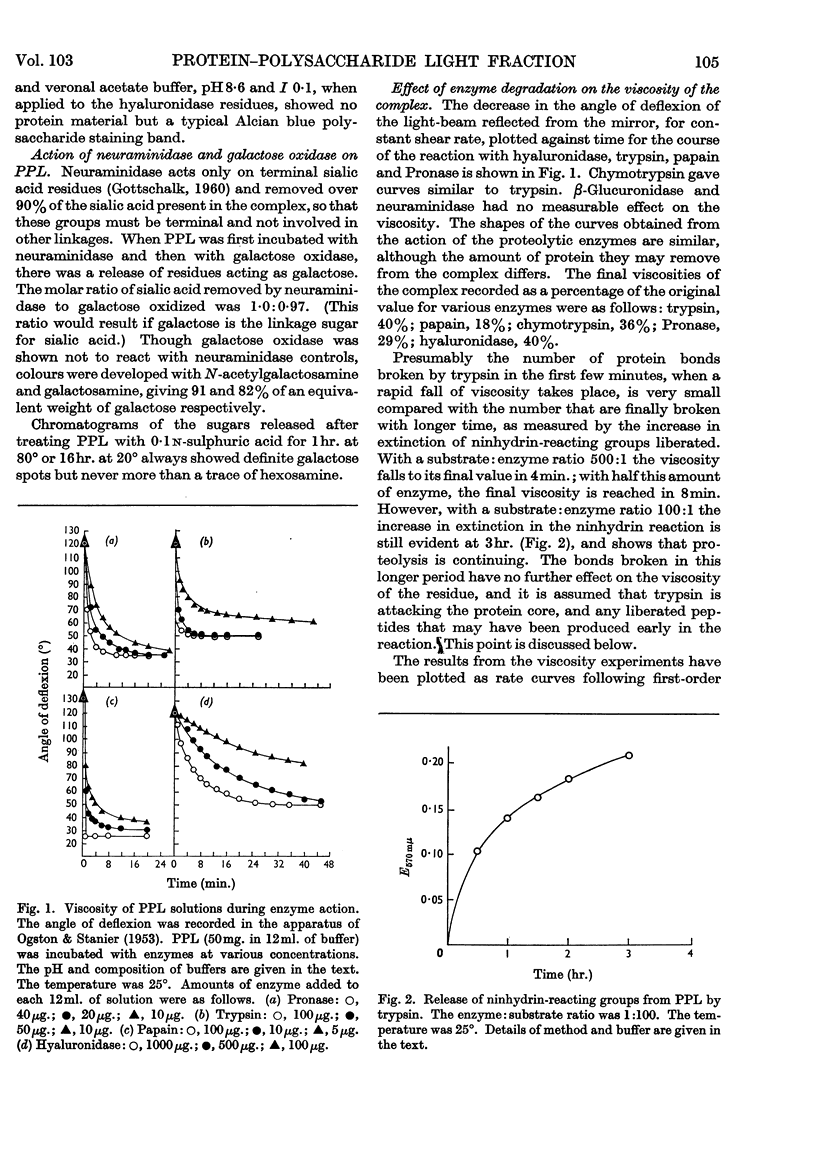
1. Fragments from enzymic degradation of protein–polysaccharide light fraction (PPL) have been analysed. 2. The time-course of action of some proteolytic enzymes and of hyaluronidase on PPL has been followed by viscometric techniques. 3. It is suggested that papain acts to produce single polysaccharide chains, whereas other proteolytic enzymes tried give evidence of twin-chain residues. 4. The molecular weight of the fragments derived from complete enzyme action on PPL supports this postulate. 5. A structure of the PPL complex is suggested.
Full text
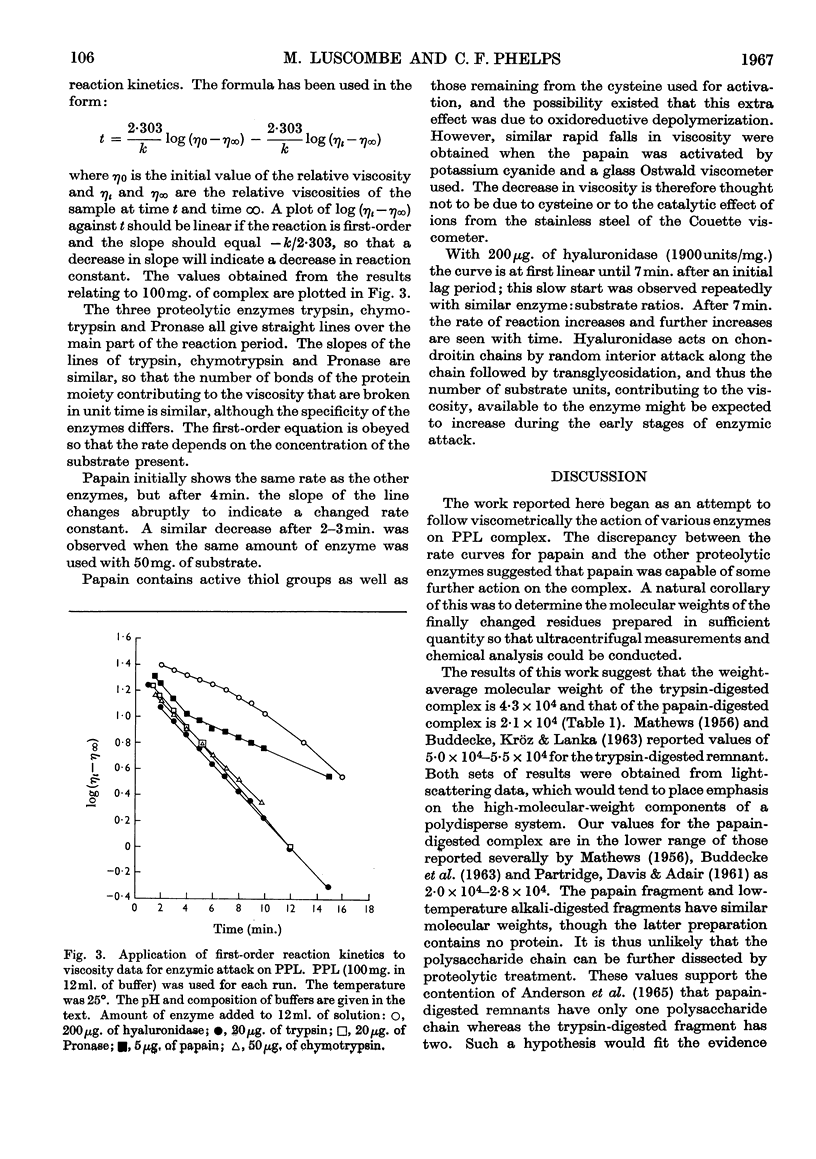
PDF

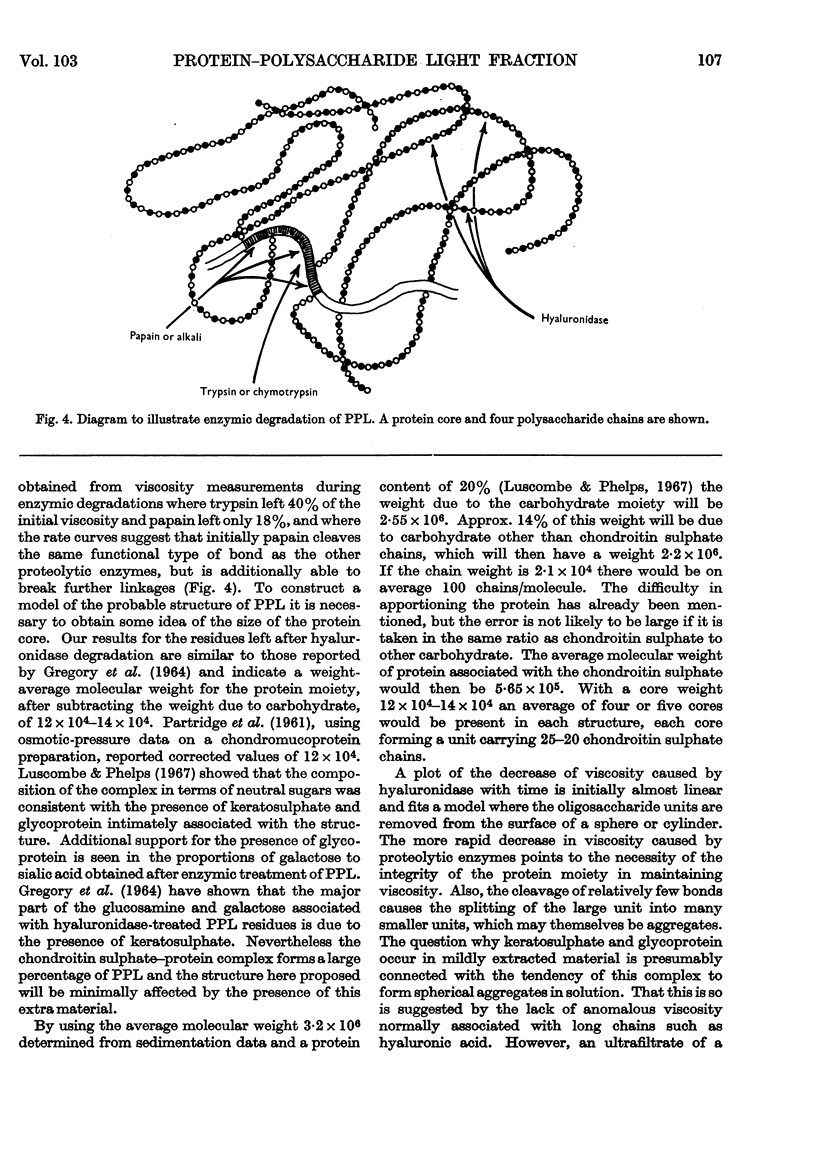

Selected References
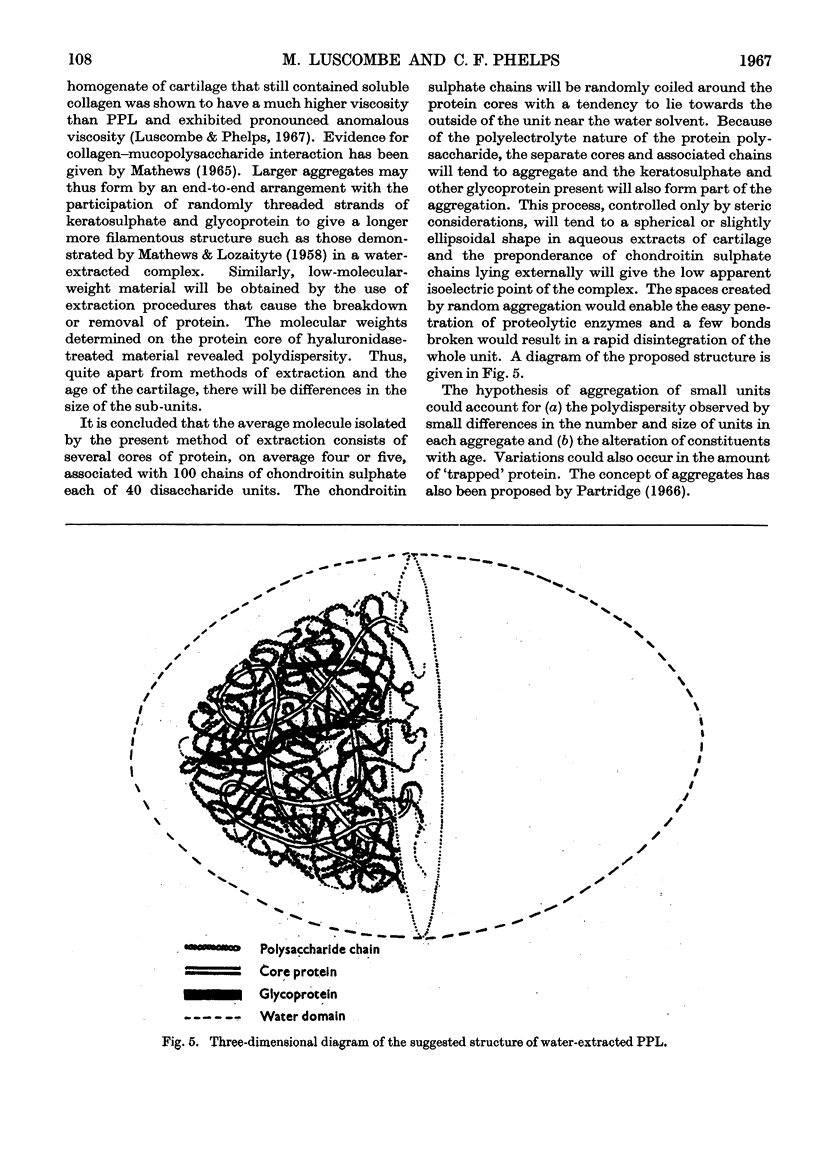
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- BUDDECKE E., KROEZ W., LANKA E. [Chemical composition and macromolecular structure of chondroitin sulfate proteins]. Hoppe Seylers Z Physiol Chem. 1963 Mar;331:196–218. doi: 10.1515/bchm2.1963.331.1.196. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D., LAURENT T. C., RODEN L. ENZYMATIC DEGRADATION OF CHONDROMUCOPROTEIN. J Biol Chem. 1964 Oct;239:3312–3320. [PubMed] [Google Scholar]
- LEWIS M. S., PIEZ K. A. SEDIMENTATION-EQUILIBRIUM STUDIES OF THE MOLECULAR WEIGHT OF SINGLE AND DOUBLE CHAINS FROM RAT-SKIN COLLAGEN. Biochemistry. 1964 Aug;3:1126–1131. doi: 10.1021/bi00896a020. [DOI] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. The composition and physicochemical properties of bovine nasal-septa protein-polysaccharide complex. Biochem J. 1967 Jan;102(1):110–119. doi: 10.1042/bj1020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B., LOZAITYTE I. Sodium chondroitin sulfate-protein complexes of cartilage. I. Molecular weight and shape. Arch Biochem Biophys. 1958 Mar;74(1):158–174. doi: 10.1016/0003-9861(58)90210-8. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. B. The molecular weight of sodium chondroitin sulfate by light scattering. Arch Biochem Biophys. 1956 Apr;61(2):367–377. doi: 10.1016/0003-9861(56)90359-9. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochem J. 1965 Sep;96(3):710–716. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., STANIER J. E. A Couette viscosimeter. Biochem J. 1953 Jan;53(1):4–7. doi: 10.1042/bj0530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 6. The constitution of the chondroitin sulphate-protein complex in cartilage. Biochem J. 1961 Apr;79:15–26. doi: 10.1042/bj0790015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M. Chondroitin sulfate-protein of bovine cartilage. Fed Proc. 1966 May-Jun;25(3):994–996. [PubMed] [Google Scholar]
- SORU E., IONESCU-STOIAN F. The purification of hyaluronate lyase on DEAE-sephadex. Biochim Biophys Acta. 1963 Mar 5;69:538–543. doi: 10.1016/0006-3002(63)91305-2. [DOI] [PubMed] [Google Scholar]


