Abstract
Lysine limitation during growth of the lysine-requiring mutant of Escherichia coli 12408 resulted in the excretion of a complex containing 60% of lipopolysaccharide, 26% of extractable phospholipid and 11% of protein. The complex was obtained from the culture filtrate in yields of about 0·5g./l. by precipitation with chloroform or gel filtration; some purification steps are described. The greater part of the phospholipid consisted of phosphatidylethanolamine, which contained four main fatty acids; two monoenoic acids and a cyclopropane acid were esterified mainly in the β-position, and a saturated acid was located mainly in the γ-position. The protein component was relatively insoluble and contained an excess of acidic over basic amino acids and little cystine. The lipopolysaccharide resembled in composition the intracellular lipopolysaccharides from rough strains of E. coli. Both protein and lipopolysaccharide constituents of the complex were serologically active; the complex was less toxic than the purified lipopolysaccharide. In the electron microscope the complex showed a mixture of particles of various sizes and shapes. Rods and hollow spheroids (diameter 12–200mμ) were most common and resembled the particles previously found surrounding cells actively excreting the complex. The chloroform-precipitated material showed a tubular lamellar structure. Soluble lipopolysaccharide prepared from the complex also consisted of hollow spheres and rods.
Full text
PDF


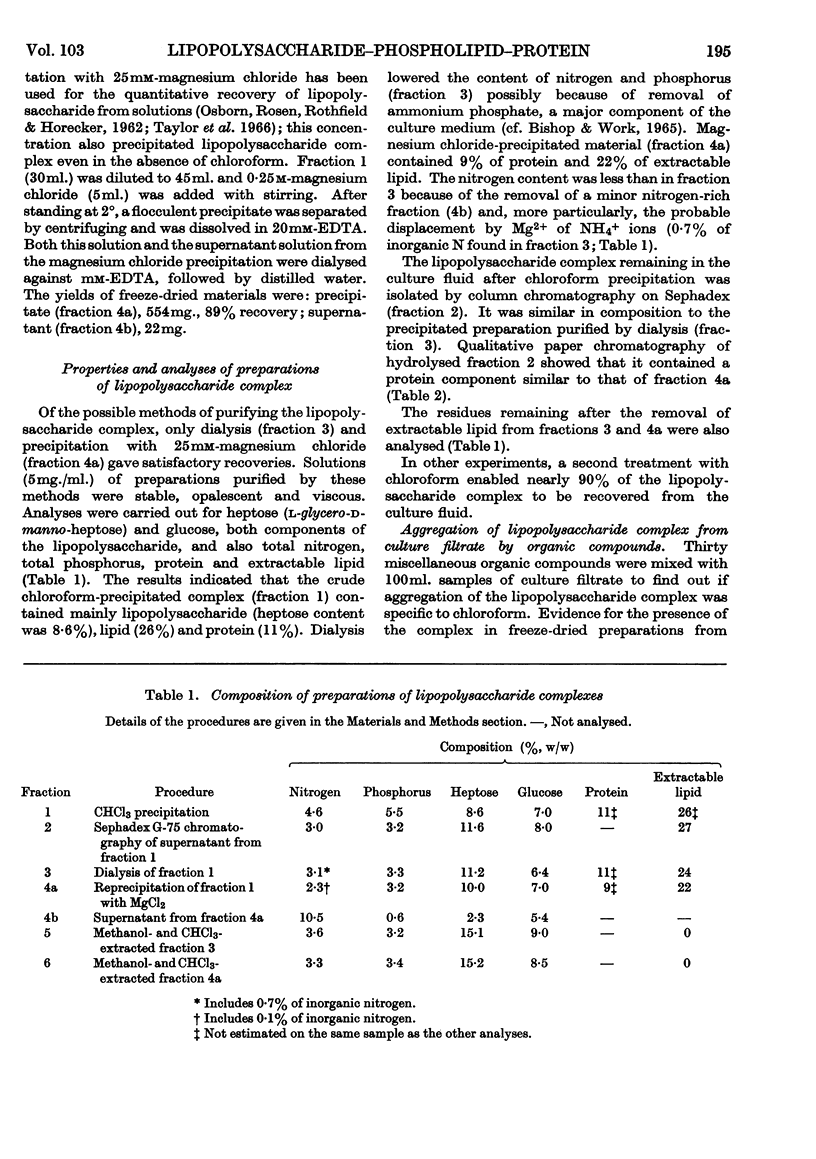



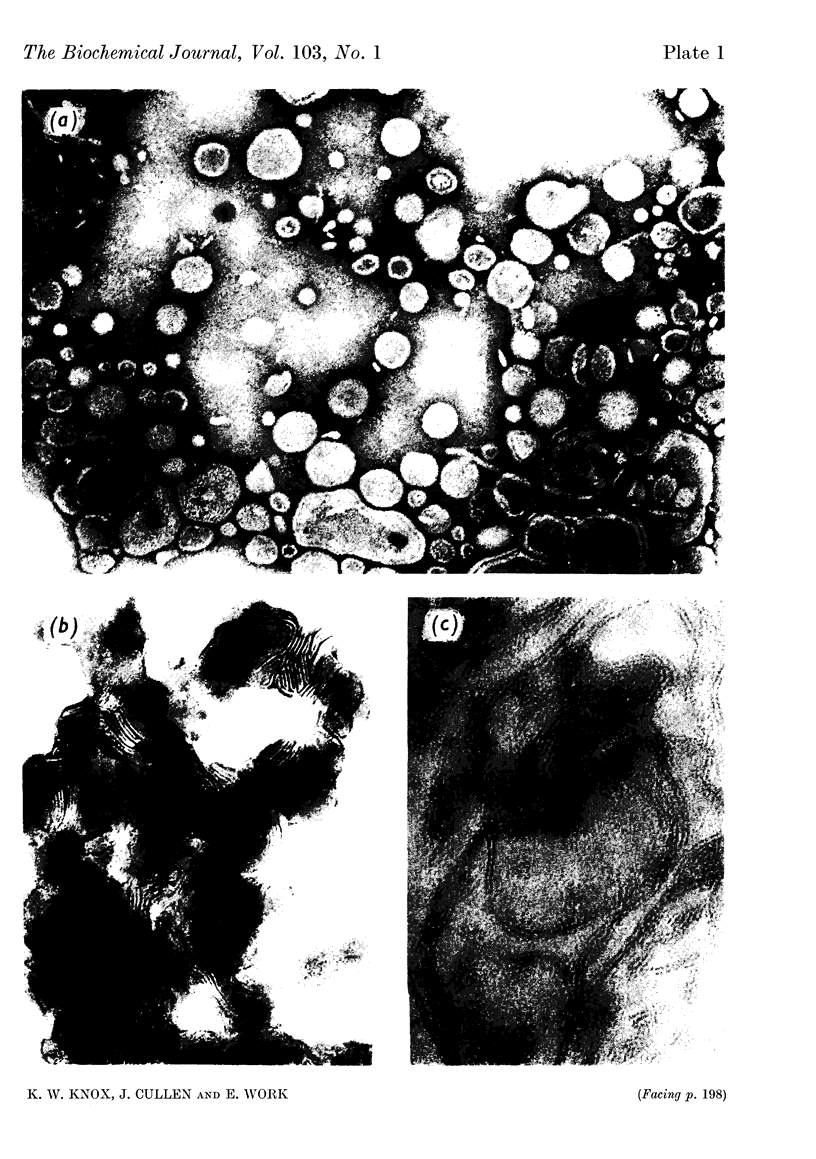




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer H., Braude A. I., Brinton C. C., Jr A study of particle sizes, shapes and toxicities present in a boivin-type endotoxic preparation. Ann N Y Acad Sci. 1966 Jun 30;133(2):450–475. doi: 10.1111/j.1749-6632.1966.tb52383.x. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1965 Aug;96(2):567–576. doi: 10.1042/bj0960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- HOMMA J. Y., SUZUKI N. "CELL-WALL PROTEIN A" OF PSEUDOMONAS AERUGINOSA AND ITS RELATIONSHIP TO "ORIGINAL ENDOTOXIN PROTEIN". J Bacteriol. 1964 Mar;87:630–640. doi: 10.1128/jb.87.3.630-640.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Suzuki N. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Ann N Y Acad Sci. 1966 Jun 30;133(2):508–526. doi: 10.1111/j.1749-6632.1966.tb52386.x. [DOI] [PubMed] [Google Scholar]
- KATES M., ADAMS G. A., MARTIN S. M. LIPIDS OF SERRATIA MARCESCENS. Can J Biochem. 1964 Apr;42:461–479. doi: 10.1139/o64-054. [DOI] [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Fatty acid synthesis in Escherichia coli. Biochem J. 1967 May;103(2):299–306. doi: 10.1042/bj1030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Some factors affecting cyclopropane acid formation in Escherichia coli. Biochem J. 1965 Sep;96(3):771–776. doi: 10.1042/bj0960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCY J. A., GLAUERT A. M. STRUCTURE AND ASSEMBLY OF MACROMOLECULAR LIPID COMPLEXES COMPOSED OF GLOBULAR MICELLES. J Mol Biol. 1964 May;8:727–748. doi: 10.1016/s0022-2836(64)80121-2. [DOI] [PubMed] [Google Scholar]
- LUEDERITZ O., RISSE H. J., SCHULTE-HOLTHAUSEN H., STROMINGER J. L., SUTHERLAND I. W., WESTPHAL O. BIOCHEMICAL STUDIES OF THE SMOOTH-ROUGH MUTATION IN SALMONELLA MINNESOTA. J Bacteriol. 1965 Feb;89:343–354. doi: 10.1128/jb.89.2.343-354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- MACFARLANE M. G. Cardiolipin and other phospholipids in ox liver. Biochem J. 1961 Jan;78:44–51. doi: 10.1042/bj0780044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINETTI G. V. CHROMATOGRAPHY OF LIPIDS ON COMMERCIAL SILICA GEL LOADED FILTER PAPER. J Lipid Res. 1965 Apr;6:315–317. [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen S. E., Bladen H. A., Hsu K. C. Electron microscopic localization of endotoxic lipopolysaccharide in gram-negative organisms. Ann N Y Acad Sci. 1966 Jun 30;133(2):279–291. doi: 10.1111/j.1749-6632.1966.tb52371.x. [DOI] [PubMed] [Google Scholar]
- NICHOLS B. W. SEPARATION OF THE LIPIDS OF PHOTOSYNTHETIC TISSUES: IMPROVEMENTS IN ANALYSIS BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1963 Aug 27;70:417–422. doi: 10.1016/0006-3002(63)90771-6. [DOI] [PubMed] [Google Scholar]
- Nowotny A., Cundy K. R., Neale N. L., Nowotny A. M., Radvany R., Thomas S. P., Tripodi D. J. Relation of structure to function in bacterial O-antigens. IV. Fractionation of the components. Ann N Y Acad Sci. 1966 Jun 30;133(2):586–603. doi: 10.1111/j.1749-6632.1966.tb52391.x. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide. Structural requirements of the phospholipid molecule. J Biol Chem. 1966 Mar 25;241(6):1386–1392. [PubMed] [Google Scholar]
- Rothfield L., Takeshita M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide: binding of transferase enzymes to a lipopolysaccharide-lipid complex. Biochem Biophys Res Commun. 1965 Aug 16;20(4):521–527. doi: 10.1016/0006-291x(65)90611-x. [DOI] [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Knox K. W., Work E. Chemical and biological properties of an extracellular lipopolysaccharide from Escherichia coli grown under lysine-limiting conditions. Biochem J. 1966 Apr;99(1):53–61. doi: 10.1042/bj0990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEENENL, DE HAAS G. H. THE SUBSTRATE SPECIFICITY OF PHOSPHOLIPASE A. Biochim Biophys Acta. 1963 Oct 22;70:538–553. doi: 10.1016/0006-3002(63)90792-3. [DOI] [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- Work E., Knox K. W., Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):438–449. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]