Abstract
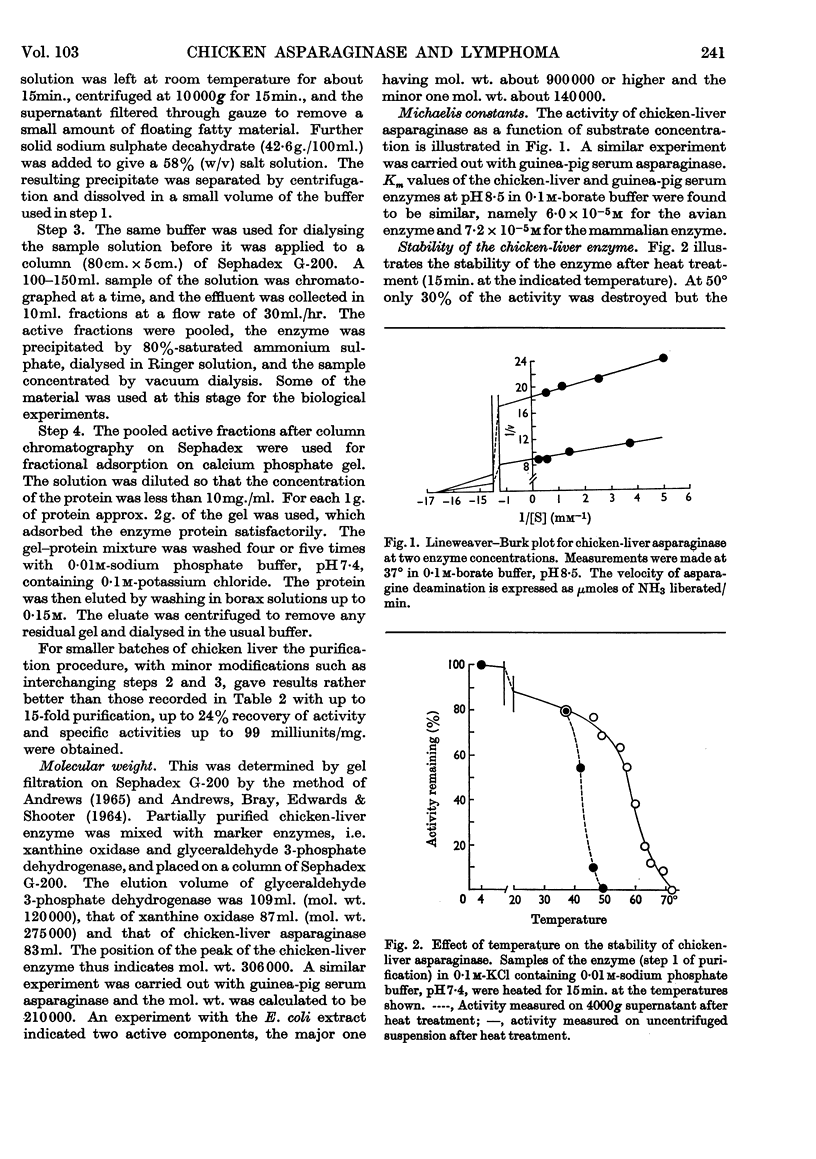
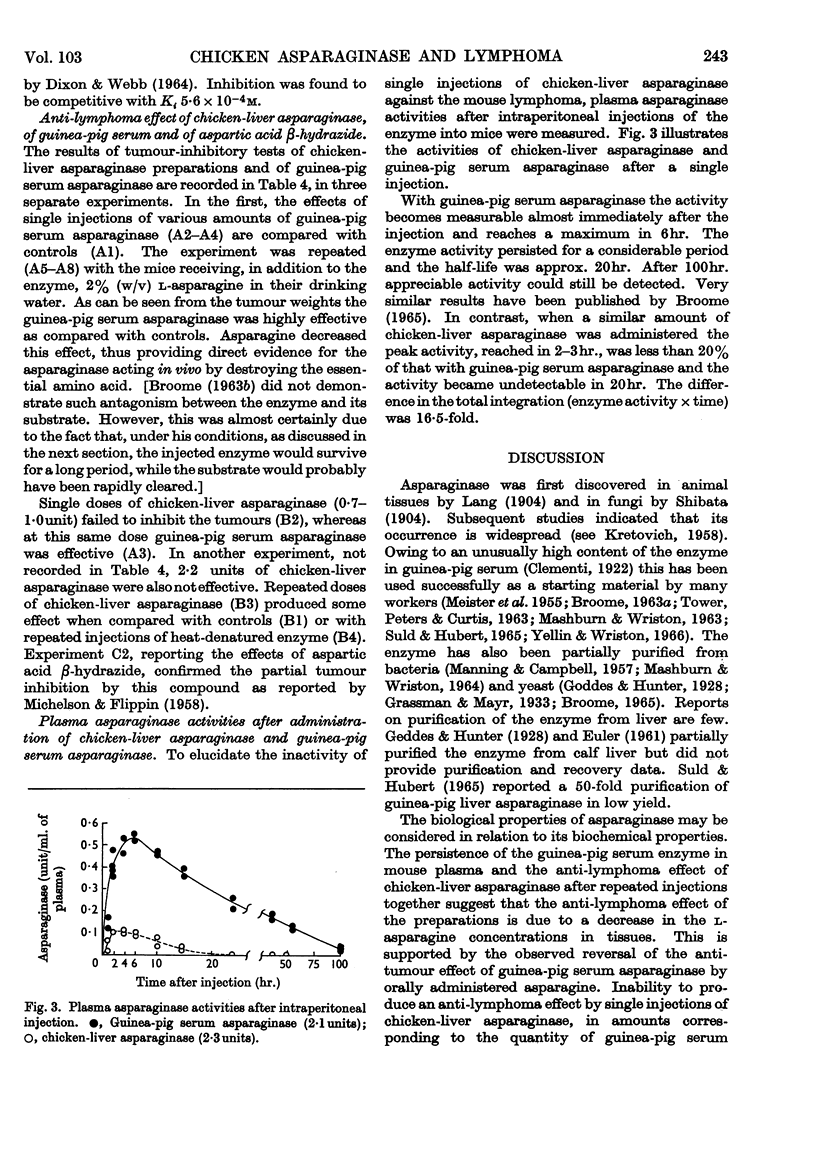
1. A procedure for partial purification of asparaginase from chicken liver is presented. 2. The bulk of the enzyme is located in the soluble fraction of chicken liver. 3. Molecular weights of chicken-liver asparaginase and of the guinea-pig serum enzyme, estimated by gel filtration, were 306000 and 210000 respectively. The Michaelis constants (Km) at 37° and pH8·5 were 6·0×10−5m and 7·2×10−5m respectively. 4. At 50° the chicken-liver enzyme was moderately stable, some activity being lost by aggregation; in dilute electrolyte solutions the activity rapidly diminished. 5. The anti-lymphoma effect of guinea-pig serum in mice carrying the 6C3HED tumour was confirmed. Chicken-liver asparaginase also showed an effect but in this case the enzyme preparation had to be administered repeatedly. 6. Guinea-pig serum asparaginase was stable for several days in mouse blood, after intraperitoneal injection, whereas chicken-liver asparaginase rapidly disappeared. 7. Aspartic acid β-hydrazide was shown to be a competitive inhibitor of chicken-liver asparaginase with Ki approx. 5·6×10−4m. In mice it produced an anti-lymphoma effect, as reported previously.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AINIS H., JAMESON E., RYAN R. M. Action of guinea pig serum and human gamma globulin on the growth of a rat tumor. Science. 1956 Nov 16;124(3229):980–981. doi: 10.1126/science.124.3229.980. [DOI] [PubMed] [Google Scholar]
- AINIS H., KURTZ H. M., KRAMER P. I., WEIMER H. E., RYAN R. M., JAMESON E. In vivo and in vitro studies of the action of guinea pig serum against the ascites form of the MurphySturm lymphosarcoma. Cancer Res. 1958 Dec;18(11):1309–1313. [PubMed] [Google Scholar]
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGEL F., BRAY R. C. The chemistry of xanthine oxidase. The problems of enzyme inactivation and stabilization. Biochem J. 1959 Sep;73:182–192. doi: 10.1042/bj0730182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYSE E. A., OLD L. J., STOCKERT E. Inhibitory effect of guinea pig serum on a number of new leukaemias in mice. Nature. 1963 May 25;198:800–800. doi: 10.1038/198800a0. [DOI] [PubMed] [Google Scholar]
- BROOME J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963 Jul;118:99–120. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOME J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. II. Lymphoma 6C3HED cells cultured in a medium devoid of L-asparagine lose their susceptibility to the effects of guinea pig serum in vivo. J Exp Med. 1963 Jul;118:121–148. doi: 10.1084/jem.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D. Antilymphoma activity of L-asparaginase in vivo: clearance rates of enzyme preparations from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst. 1965 Dec;35(6):967–974. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. A., Bergel F. The chemistry of xanthine oxidase. 9. An improved method of preparing the bovine milk enzyme. Biochem J. 1964 Feb;90(2):350–353. doi: 10.1042/bj0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBUT P. A., KRAEMER W. H., PILLEMER L. The effects of components of guinea pig serum on lymphosarcoma 6C3HED in C3H mice. Blood. 1958 Aug;13(8):732–739. [PubMed] [Google Scholar]
- JAMESON E., AINIS H., RYAN R. M. The inhibition by guinea pig serum of the growth of the Murphy-Sturm lymphosarcoma. Cancer Res. 1958 Aug;18(7):866–868. [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953 Dec;98(6):565–582. doi: 10.1084/jem.98.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: histological mechanism of the regression: tests for effects of guinea pig serum on lymphoma cells in vitro: discussion. J Exp Med. 1953 Dec;98(6):583–606. doi: 10.1084/jem.98.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONITZER K., VOIGT S. [Direct determination of ammonium in blood and tissue extracts by means of the phenol by chlorite reaction]. Clin Chim Acta. 1963 Jan;8:5–11. doi: 10.1016/0009-8981(63)90192-x. [DOI] [PubMed] [Google Scholar]
- KRETOVICH W. L. The biosynthesis of dicarboxylic amino acids and enzymic transformations of amides in plants. Adv Enzymol Relat Subj Biochem. 1958;20:319–340. doi: 10.1002/9780470122655.ch10. [DOI] [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L., Jr The asparagine deamidase of Bacillus coagulans and Bacillus stearothermophilus. Can J Microbiol. 1957 Dec;3(7):1001–1009. doi: 10.1139/m57-111. [DOI] [PubMed] [Google Scholar]
- MASHBURN L. T., WRISTON J. C., Jr TUMOR INHIBITORY EFFECT OF L-ASPARAGINASE FROM ESCHERICHIA COLI. Arch Biochem Biophys. 1964 May;105:450–452. doi: 10.1016/0003-9861(64)90032-3. [DOI] [PubMed] [Google Scholar]
- MEISTER A., LEVINTOW L., GREENFIELD R. E., ABENDSCHEIN P. A. Hydrolysis and transfer reactions catalyzed by omega-amidase preparations. J Biol Chem. 1955 Jul;215(1):441–460. [PubMed] [Google Scholar]
- MICKELSON M. N., FLIPPIN R. S. Effects of certain amino acid derivatives on microorganisms and sarcoma 180. J Natl Cancer Inst. 1958 Mar;20(3):495–512. [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. The L-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous L-amino acid oxidase. Arch Biochem. 1950 Nov;29(1):190–209. [PubMed] [Google Scholar]
- STEIN W. H., MOORE S. The free amino acids of human blood plasma. J Biol Chem. 1954 Dec;211(2):915–926. [PubMed] [Google Scholar]
- SULD H. M., HERBUT P. A. GUINEA PIG SERUM AND LIVER ASPARAGINASES. PURIFICATION AND ANTITUMOR ACTIVITY. J Biol Chem. 1965 May;240:2234–2241. [PubMed] [Google Scholar]
- TALLAN H. H., MOORE S., STEIN W. H. Studies on the free amino acids and related compounds in the tissues of the cat. J Biol Chem. 1954 Dec;211(2):927–939. [PubMed] [Google Scholar]
- TOWER D. B., PETERS E. L., CURTIS W. C. Guinea pig serum L-asparaginase. Properties, purification, and application to determination of asparagine in biological samples. J Biol Chem. 1963 Mar;238:983–993. [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Yellin T. O., Wriston J. C., Jr Antagonism of purified asparaginase from guinea pig serum toward lymphoma. Science. 1966 Feb 25;151(3713):998–999. doi: 10.1126/science.151.3713.998. [DOI] [PubMed] [Google Scholar]


